Uranium is a naturally occurring radioactive chemical element. It was formed in super novae explosions about 6.6 billion years ago. In its pure form it is a silvery colored heavy metal. It is 70% denser than lead but not quite as dense as gold and will burn in a powdered form. A little softer than steel, it is malleable, ductile, paramagnetic, weakly electropositive and a poor conductor of electricity. It will oxidize in air and can be dissolved by acids.
Uranium is a common element that is present in low concentrations of a few parts per million in soil, rocks and water. It is thought to be forty times as abundant as silver. Uranium can react with most of the non-metallic elements and their compounds. Hundreds of minerals contain uranium. Uraninite, autunite, uranphane, torbernite, coffinite and pitchblende are common ores of uranium with uraninite being the most abundant.
The most common and stable form of uranium found in nature is an oxide called triuranium. Each molecule contains 3 uranium atoms and 8 oxygen atoms. Uranium dioxide which contains 1 atom of uranium and 2 atoms of oxygen is the most common nuclear fuel used in nuclear reactors.
The symbol for uranium is the letter “U”. The nucleus of the uranium atom contains 92 protons which is the atomic number. In the periodic table, uranium is a member of the actinoids group of transitional elements. Some of the actinoids do not occur in nature and are created by nuclear transmutation processes.
The nucleus of the uranium atom also contains from 141 to 146 neutrons creating six different isotopes referred to as U-233 to U-238. All of the isotopes are unstable and radioactive. Most (99.274 %) of natural uranium is in the form of the U-238 isotope. A little (.720 %) natural uranium is the U-235 isotope and a tiny (.005%) amount is U-234.
Uranium decays slowly by emission of particle consisting of two protons and two neutrons also known as an alpha particle. The half-life of U-238 is 4.5 billion years and the half-life of U-235 is 700 million years. Uranium, thorium and plutonium are the three fissile elements which can disintegrate into lighter elements. Isotopes of these elements go through radioactive decay processes where a series of elements create other elements until a stable non-radioactive element is reached.
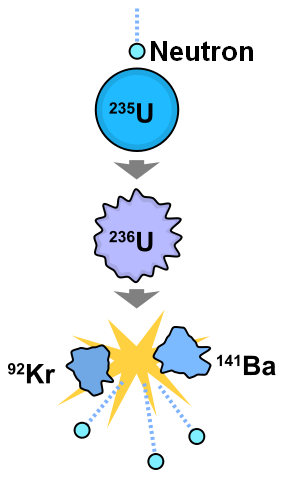
U-235 is the only naturally occurring elementary isotope that is capable of sustaining a nuclear chain reaction. When bombarded with slow neutrons, U-235 converts temporarily to U-238 which immediately disintegrates into the noble gas krypton, Kr-85, the alkaline earth metal barium, Ba-141creating heat and release more neutrons. The K5-85 and Ba-141 are unstable and also decay. If more U-235 atoms are hit by these neutrons, a chain reaction can occur. It is thought that the heat generated by the disintegration of U-235 provides most of the heat in the interior of the Earth that keeps the core liquid and drives plate tectonics.