Some elements are transmuted to other elements in nuclear reactors. This process is called nuclear transmutation. One of the options discussed for disposal of some radioactive materials is to intentionally convert them to other less radioactive elements in a reactor or with another source of radiation.
The set of elements referred to as “actinides” are 15 metallic elements that range in atomic number from 89 to 103. All the actinides are radioactive. Uranium and thorium are naturally occurring members and there is a little natural plutonium as well. These three actinides are by far the most abundant of all the actinides. Radioactive decay of natural uranium will temporarily produce small amounts of actinium (for which the actinide row of the periodic table is named) and protactinium. Other actinides such as neptunium, americium, curium, berkelium and californium can be produced in tiny amounts from transmutation processes in uranium ores. The remaining five actinides are einsteinium, fermium, mendelevium, nobelium and lawrencium. They are synthetic elements that are only produced by human activities.
The hundreds of light water reactors scattered across the world produce waste in the form of uranium, plutonium, actinides and fission products. Uranium constitutes about 96 percent of the waste and contains about one percent of U-235 which can be refined back into fuel. Another one percent of the waste is plutonium that can be used as a fuel. Actinides are about one tenth of one percent of the waste. Neptunium and americium are each about one half of the actinide waste with a few additional percent of curium. These three are very radioactive and toxic. There are also rare earth elements in the waste which are chemically similar to the actinides but mostly non-radioactive. The final 3 percent of the nuclear waste from reactors contains a mix of fission products which include iodine, technetium, neodymium, zirconium, molybdenum, cerium, cesium, ruthenium, palladium and other elements.
The first step in transmutation of actinides in waste is to separate them from lanthanides which are not radioactive. This is very difficult because they are chemically similar. The lanthanides have to be removed because they efficiently absorb neutrons and would prevent the transmutation of the actinides. Once separated, the actinides are bombarded with neutrons inside a reactor or linear accelerator and forced to fission which produces an assortment of fission products. The reason that this is attractive with respect to waste disposal is the fact that most of the actinides in the waste have half-lives of thousands of years as opposed to the half-lives measured in decades in most of the radioactive fission products. Other fission produces are stable elements.
Iodine-129 is a dangerous radioisotope produced by fission processes. It will dissolve easily in water and move through the environment. It is concentrated in the human thyroid when ingested and can cause thyroid cancer. Subjecting iodine-129 to neutron bombardment will convert it to stable xenon. Similarly, technetium-99 is very soluble, mobile and toxic. Neutron bombardment will convert it first to technetium-100 which immediately decays to stable ruthenium.
While theoretically a method to dispose or at least make radioactive waste less long lived, nuclear transmutation will require extensive research and development of practical solutions to a number of technical challengers to be a serious option for treating large quantities of nuclear waste.
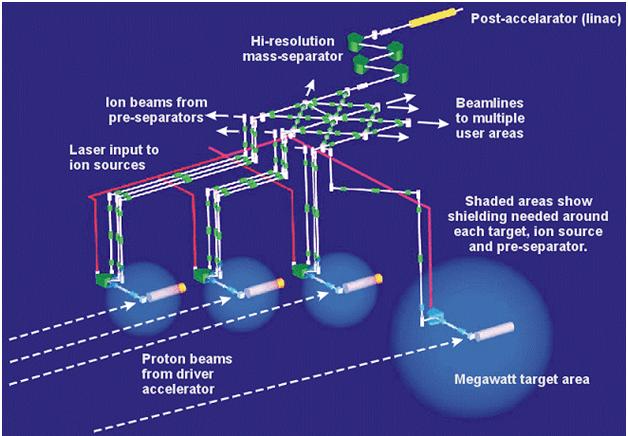
Proposed French nuclear transmutation system from irfu.cea.fr: