Bulgaria has shortlisted five companies to participate in the Belene nuclear power plant project. The applicants included three to be a strategic investor and two to be an equipment supplier. World-nuclear-news.org
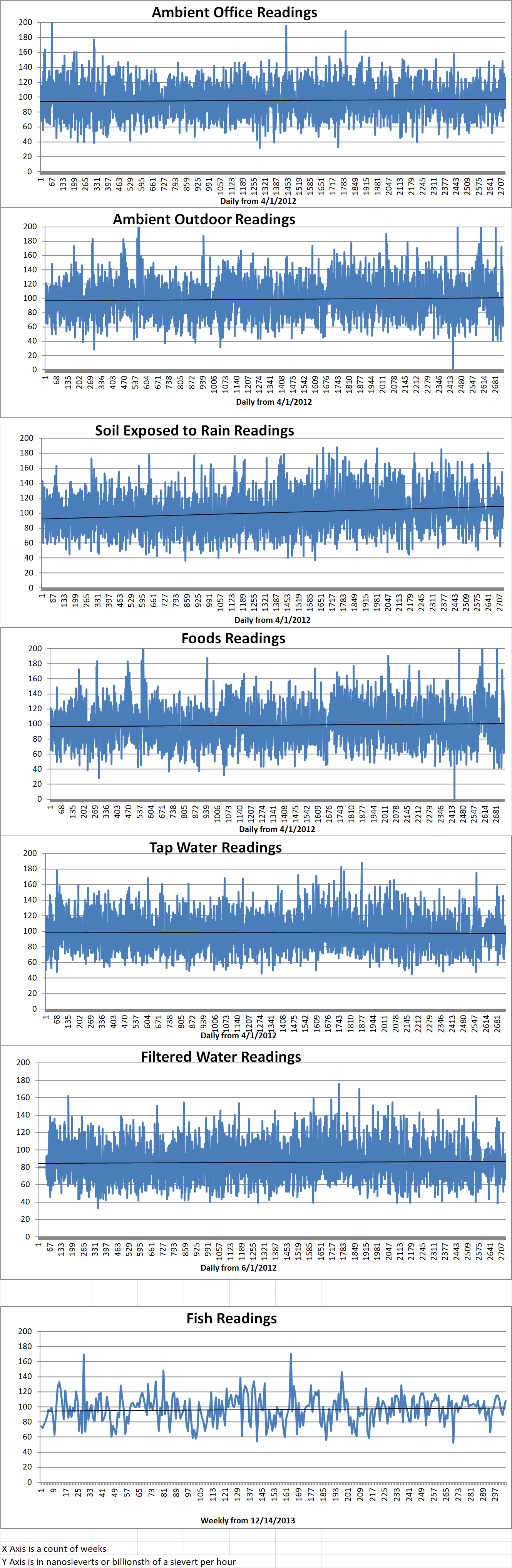
Ambient office = 126 nanosieverts per hour
Ambient outside = 97 nanosieverts per hour
Soil exposed to rain water = 97 nanosieverts per hour
Avocado from Central Market =87 nanosieverts per hour
Tap water = 106 nanosieverts per hour
Filtered water = 91 nanosieverts per hour
NRC gives permit for next-gen factory-built nuclear reactor ksl.com
Kansai Electric to Halt Takahama Nuclear Reactors, Mainichi Says Bloomberg.com
Faith leaders, heed pope’s call on nuclear weapons heraldneet.com
How Trump’s North Korea nuclear talks gambit came undone washingtonpost.com

Ambient office = 126 nanosieverts per hour
Ambient outside = 115 nanosieverts per hour
Soil exposed to rain water = 118 nanosieverts per hour
Sugarbee apple from Central Market = 880 nanosieverts per hour
Tap water = 105 nanosieverts per hour
Filtered water = 95 nanosieverts per hour
Dover sole – Caught in USA = 108 nanosieverts per hour
Part 2 of 2 Parts (Please read Part 1 first)
Bazant says “We carefully measure the composition of all the stuff going in and out. This really opened up a new direction for our research.” His team have begun to focus on separation processes that could be useful with respect to public health. They are also interested in concentrating contaminants that have high value either for resale or to offset disposal costs for the contaminated water.
While the new MIT process can remove salt from water, it requires too much energy to be competitive with other desalinization processes. The energy cost is much lower when the new technique is used to selectively separate specific ions from dilute streams of waters such as those found in nuclear power plant cooling systems. Bazant says that his new technique is cost effective for this application. This particular application also satisfies both of the goals of team: producing high value materials and protecting public health. The ability to scale-up the MIT decontamination system is also very important. One big nuclear power plant can circulate about ten million cubic meters of water in a year through its cooling system.
To test their new process, the MIT team made use of simulated nuclear cooling wastewater. The simulated wastewater was based on a recipe developed by Mitsubishi Heavy Industries which is a sponsor of the MIT research and is a major contractor for nuclear power plants. After a three-stage separation process, the MIT test resulted in the removal of ninety-nine and one-half percent of the cobalt radionuclides in the water. About forty-three percent of the water in the test was clean enough to be reused. If the cleanup level is reduced to ninety-eight and one-third percent of the cobalt radionuclides removed, two-thirds of the water could be recycled.
There are many possible applications for the new MIT technique. Bazant says that nuclear wastewater separation is “one of the first problems we think we can solve [with this method] that no other solution exists for.” There are no other practical, continuous, economical methods for separating out the radioactive isotopes of cobalt and cesium. These are two major contaminants of nuclear wastewater.
The new MIT technique will certainly be useful for the routine cleanup of contaminated cooling water in nuclear power plants. It can also be a big help in dealing with more extreme situations such as the millions of gallons of contaminated water being stored at the site of the Fukushima nuclear disaster. The Fukushima operators are running out of room to store the accumulating contaminated ground water and they have been talking about dumping it into the Pacific Ocean untreated.
Bazant says that while his new technique has only been tested at small scales, he believes that large-scale decontamination systems may be possible within a few years.
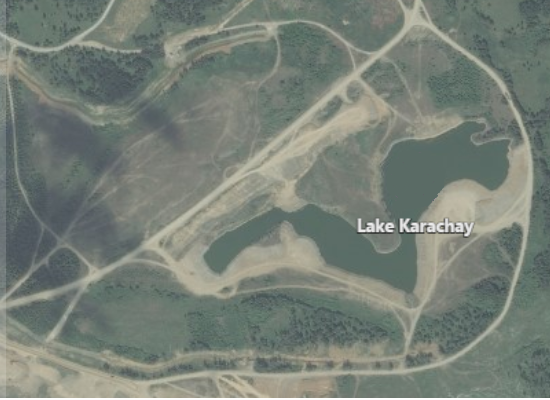
There are some bodies of water that are horribly contaminated with radioactive materials such as Lake Karachay in the Mayak region of southern Russia. This lake was a dumping ground for radioactive waste from the Soviet and Russian nuclear weapons development programs. It is considered to be the most polluted place in the world. If a person spent an hour on the shore of this lake, the radiation would kill them. Is it possible the new contamination mitigation system developed by Bazant and his team at MIT could be utilized to decontaminate Lake Krarchay?
GE Hitachi Nuclear Energy Completes Reactor Decommissioning Project in Sweden powermag.com
China, EU Powers Agree to Help Save Iran Nuclear Deal financial tribune
Bulgaria shortlists GE, others for nuclear plant contract washingtonpost.com
China Wants Its Very Own Nuclear Aircraft Carrier (And Wants Russian Help) news.yahoo.com
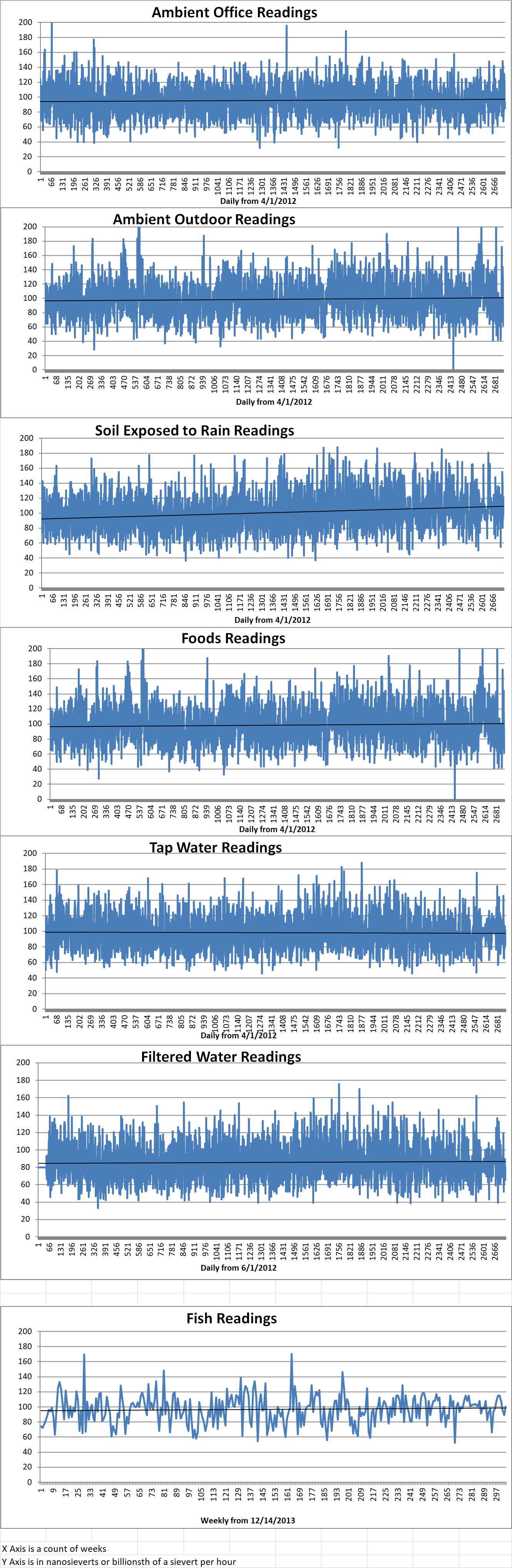
Ambient office = 85 nanosieverts per hour
Ambient outside = 115 nanosieverts per hour
Soil exposed to rain water = 116 nanosieverts per hour
Blueberry from Central Market = 63 nanosieverts per hour
Tap water = 84 nanosieverts per hour
Filtered water = 71 nanosieverts per hour

Part 1 of 2 Parts
I have often blogged about problems with the disposal of spent nuclear fuel. There are other nuclear waste issues such as dealing with the decontamination of surface and ground water polluted with radioactive materials. There is great interest in developing methods of removing radioactive contaminants from bodies of water. Today I am going to blog about a new system developed by MIT.
While the construction of new nuclear power plants has slowed in the Americas and in Europe, Russia and China are still dedicated to massive construction programs for nuclear power reactors for domestic use and export. A major benefit of nuclear power is that the operation of nuclear power plants does not emit any greenhouse gases. This can help mitigate climate change. (There are still carbon emissions associated with the creation of nuclear fuel and the construction of the nuclear power plants.)
Nuclear power plants require huge amounts of cooling water. This cooling water is contaminated by radioactive isotopes with long half-lives which poses a problem for long term disposal. In addition, the Fukushima nuclear disaster in 2011 in Japan generated and is still generating millions of gallons of contaminated water that must be dealt with.
Now a team at MIT has developed a solution for dealing with huge amounts of contaminated water. Their new system can concentrate the contaminants in reactor cooling water. This allows most of the cooling water to be recycled in the plant’s cooling system. Their new system design was published in the journal Environmental Science and Technology. The paper was authored by Martin Bazant who is the E.G. Roos Professor of Chemical Engineering as well as a professor of mathematics. He was assisted by graduate students named Mohammad Alkhadra and Huanhuan Tian as well as postdocs Kameron Conforti and Tao Gao.
The new MIT system is based on a process called “shock electrodialysis.” It utilizes an electrical field which generates a deionization shockwave in water. This shockwave forces the electrically charged particles (ions) in the water to one side of a tube that is full of a charged porous material. This allows a concentrated stream of contaminating particles to be removed from the water. The MIT team discovered that two radionuclide contaminants consisting of isotopes of cobalt and cesium can be selectively separated from water that also contains boric acid and lithium. Once the contaminants have been removed, the water can be recycled.
Bazant and his team first developed the shock electrolysis process to remove salt from water. Their first scalable prototype was constructed four years ago for this specific purpose. The team is now focusing on the more specific purpose of removing nuclear contamination from power plant wastewater. This application could assist in the improvement of the economics of nuclear power plants. It would also reduce the environmental impact of nuclear power. They are continuing to develop the new technology for other applications such as removing lead from drinking water.
The new MIT system is cheap and easily scales to larger sizes. In principle, it should be able to deal with a wide variety of contaminants. Bazant says, “It’s a single device that can perform a whole range of separations for any specific application.”
In early research, the MIT team measured the electrical conductivity of the water in order to determine exactly how much of the contamination had been removed. Since then, other methods for detecting and quantifying the details of what is in the concentrated contaminants and what is left in the cleaned water have been developed.
Please read Part 2
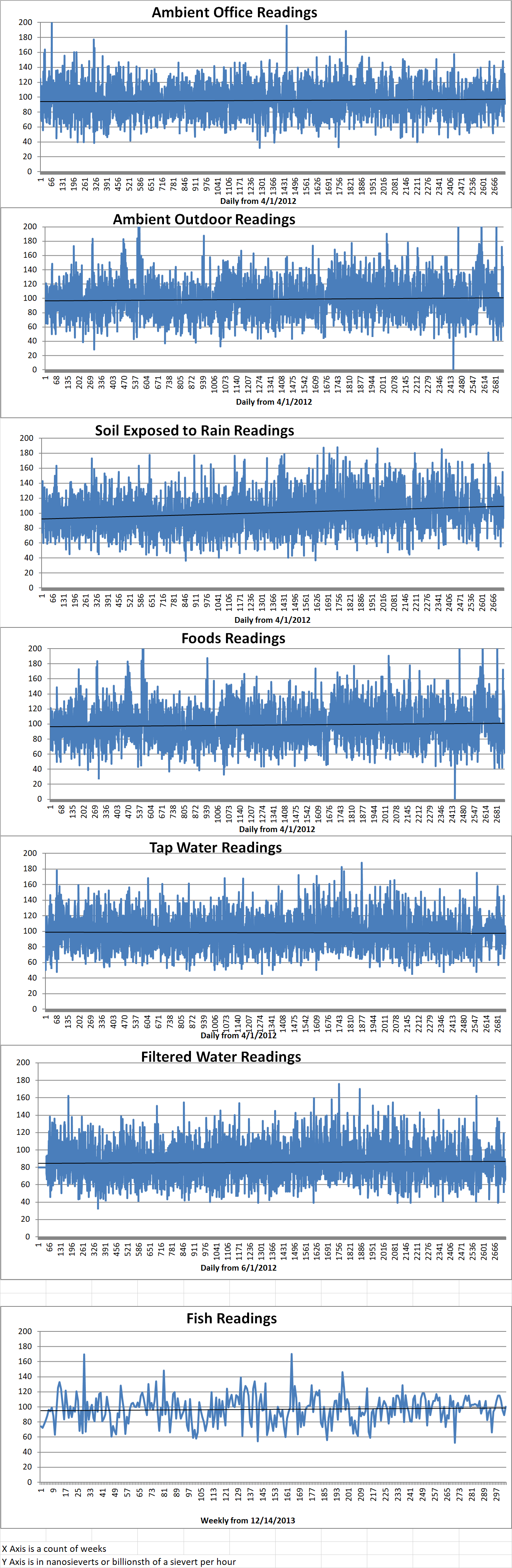
Ambient office = 91 nanosieverts per hour
Ambient outside = 99 nanosieverts per hour
Soil exposed to rain water = 101 nanosieverts per hour
Avocado from Central Market = 68 nanosieverts per hour
Tap water = 101 nanosieverts per hour
Filtered water = 69 nanosieverts per hour