Ads Flood Airwaves As Debate Continues Over Nuclear Bailout Bill wksu.org
Trump and Kim’s Cozy Relationship Makes Nuclear Talks Tougher Bloomberg.com
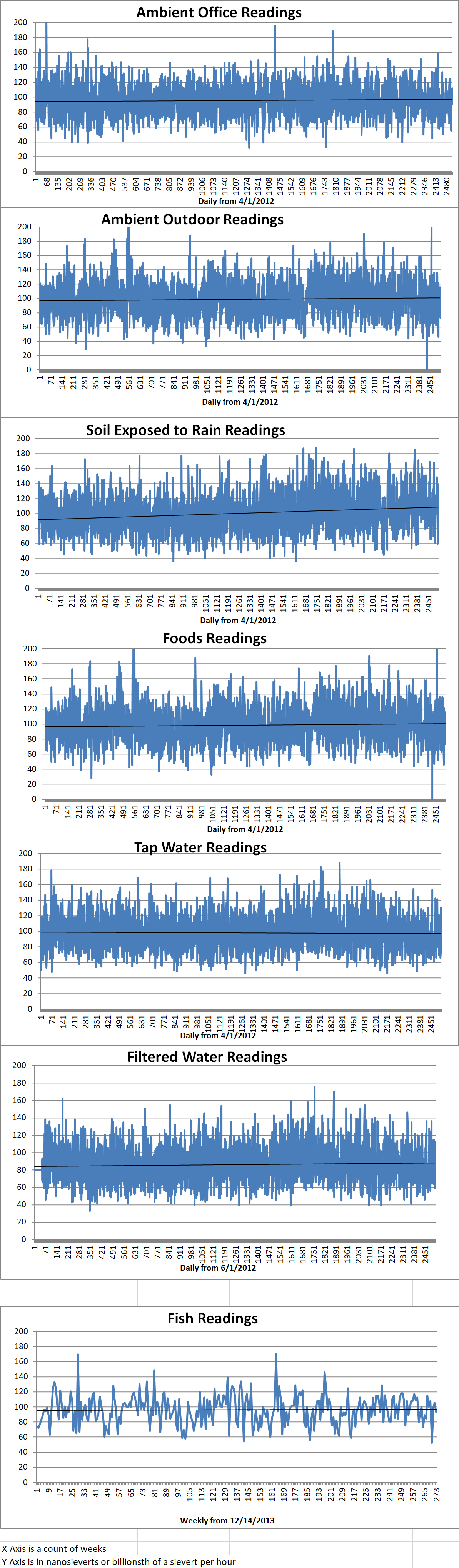
Ambient office = 112 nanosieverts per hour
Ambient outside = 95 nanosieverts per hour
Soil exposed to rain water = 94 nanosieverts per hour
Tomato from Central Market = 73 nanosieverts per hour
Tap water = 89 nanosieverts per hour
Filtered water = 73 nanosieverts per hour
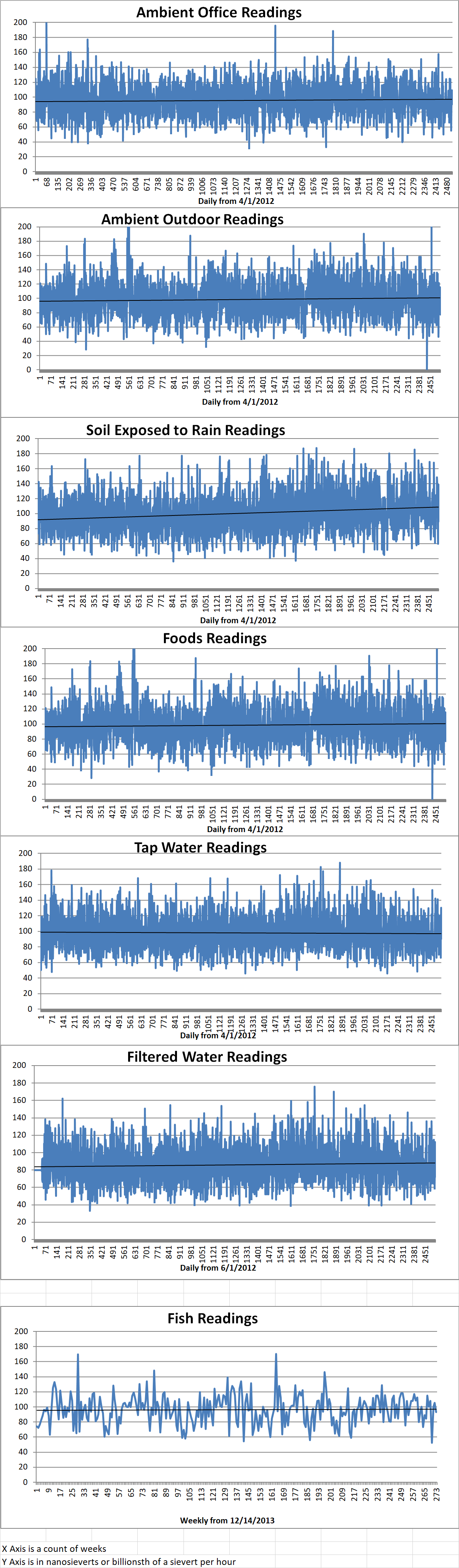
Ambient office = 98 nanosieverts per hour
Ambient outside = 89 nanosieverts per hour
Soil exposed to rain water = 90 nanosieverts per hour
Carrot from Central Market = 77 nanosieverts per hour
Tap water = 108 nanosieverts per hour
Filtered water = 91 nanosieverts per hour
Dover sole – Caught in USA = 93 nanosieverts per hour

A research team from the Department of Energy’s Oak Ridge and Lawrence Berkeley National Laboratories, the University of California – Berkeley, and the University of South Florida have developed a low-cost polymer adsorbent that can selectively bind to dissolved uranium in water. A report on the new process has just been published in the Nature Communications journal. This new process could significantly lower the cost and raise the efficiency of extracting uranium from the oceans of the world for sustainable nuclear energy production.
Ilja Popov works in the ORNL’s Chemical Sciences Division. He is one of the coauthors of the report. He said, “Our approach is a significant leap forward. Our material is tailor-made for selecting uranium over other metals present in seawater and can easily be recycled for reuse, making it much more practical and efficient than previously developed adsorbents.”
Microorganisms that have an affinity for iron were an inspiration for this research. Such organisms use natural compounds called “siderophores” to draw nutrients such as iron from their hosts. Popov said, “We essentially created an artificial siderophore to improve the way materials select and bind uranium.” His team used computer modeling and laboratory experimentation to identify a novel functional group known as “H2BHT” (2,6-bis[hydroxy(methyl)amino]-4-morpholino-1,3,5-triazine) that preferentially selects uranyl ions (water-soluable uranium) over other metal ions from other elements such as vanadium which are found in seawater.
The discovery of this new material is supported by the promising behavior of a proof-of-principle H2BHT polymer adsorbent in the lab. Uranyl ions are readily adsorbed to the surface of the polymer fibers because of the unique chemical properties of H2BHT. One of the benefits of the new polymer is that it increases the “storage space” on the polymer fibers for uranium. H2BHT is highly selective for uranium and it is recyclable. It is more efficient than any other such method for recovering uranium. A practical method for recovering uranium from seawater could provide fuel for nuclear power reactors for thousands of years at our current rate of consumption.
There are uranium deposits on the floor of the oceans which are constantly being eroded to supply uranyl ions to seawater. It is estimated that there are about three milligrams of uranium per ton of seawater. Even at a very dilute concentration, the oceans of the world contain an estimated four billion tons of uranium. This is roughly a thousand times more uranium than sources on land.
Researchers have been working on the creation of efficient uranium adsorbents to extract uranium from seawater for over fifty years, but the goal has been elusive. Alexander Ivanov does computational studies of H2BHT at the ORNL. He said, “The goal is to develop efficient adsorbent materials at a low cost that can be processed using mild conditions to recover uranium, and also reused for multiple extraction cycles.”
DoE’s Office of Nuclear Energy’s Fuel Cycle Research and Development has been supporting this research. The focus of the team has been to identify the factors that selectively influence and increase the volume of uranium that can be extracted by new materials. Previously reported studies of uranium extraction utilizing amidoxime-based polymers revealed that amidoxime has a greater affinity for vanadium than uranium that has been difficult to overcome. The development of H2BHT which is not based on amidoxime has a better ability to selectively target uranium in seawater. Popov said, “The result is that amidoxime-based materials, the current front-runners for commercially available adsorbents, fill up more quickly with vanadium than uranium, which is difficult and costly to remove.”
The old amidoxime-based polymers require concentrated acidic solutions to wash out the vanadium which increases the cost. The result is caustic waste streams and degradation of the polymer fibers which limits the number of times they can be recycled. Popov said, “To work as a scaled-up concept, ideally, unwanted elements would not be adsorbed or could easily be stripped during processing and the material reused for several cycles to maximize the amount of uranium collected.”
In contrast, the process utilizing H2BHT is carried out with mild basic solutions and the fibers can be recycled many times without degradation. The lower cost over the amidoxime-based process and the lesser environmental impact means that H2BHT may be the key to practical uranium extraction from seawater without the environmental devastation caused by mining uranium on land.
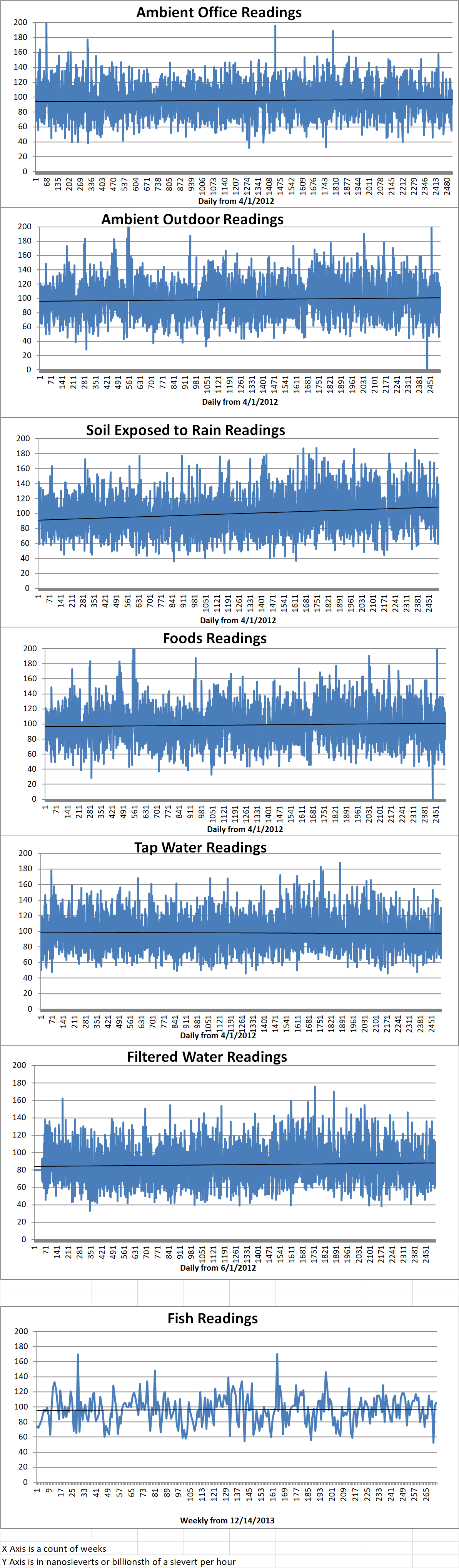
Ambient office = 93 nanosieverts per hour
Ambient outside = 135 nanosieverts per hour
Soil exposed to rain water = 139 nanosieverts per hour
Broccoli from Central Market = 116 nanosieverts per hour
Tap water = 90 nanosieverts per hour
Filtered water = 72 nanosieverts per hour

Recently, I wrote about a cube of uranium that was donated to a scientist anonymously. It turned out that it was confiscated from a German laboratory at the end of World War II. Several years ago, a mislabeled sample of radioactive material was discovered in a college chem lab. No one was sure where it came from. Apparently there are a lot of samples of unrecognized radioactive materials floating around. Another one of these orphan samples was just discovered recently at a high school in Ohio.
Zahn’s Corner Middle School is located in the town of Piketon which is about eighty miles east of Cincinnati. The school has about 320 students. A small canister of powered enriched uranium was discovered at the school. Trances of Neptunium 237 were also found. Both of these materials are highly radioactive and can cause cancer with prolonged exposure.
Scioto Valley Local School District Superintendent Todd Burkitt ordered that the school be closed last Monday. He said, “Even the last couple of hours have been very hectic. There’s just not a playbook in how we deal with this. We’re kind of writing the script as we go. We’re not going to take any chances on someone’s child. We just won’t do that.” He also said that the school may remain closed while they investigate. The state department of education said that the students at the school had already put in the required hours for the year. Fortunately, if a long closure is required while the investigation proceeds, it will not be necessary for any of the students to make up missed days.
It is unclear exactly where the radioactive materials came from. It has been suggested that the source may be the nearby Portsmouth Gaseous Diffusion Plant which is only two miles from the school. This facility enriched uranium for the U.S. government. Both reactor grade uranium for use by the United States Atomic Energy program and weapons-grade uranium for use in U.S. nuclear weapons were enriched there. The facility stopped enriching uranium in 2001.
Because of the discovery of the radioactive materials, the school now requires an environmental cleanup under the supervision of the Department of Energy (DoE). A DoE official told a local television station that “Routine air samples in the area of DOE Portsmouth Gaseous Diffusion Plant in Piketon revealed trace amounts of two radiological isotopes that were more than one thousand to ten thousand times below the established threshold of public health concern. DOE treats all detections seriously—even those that are at such low levels.” The DoE statement went on to say that the Department was “committed to the safety, health and protection of our workforce, the general public and the environment at all our sites.” The DoE is planning on commissioning an “independent third party to perform an additional analysis of the air and ground readings to properly assess the situation.” The DoE is “confident that those findings will allay any cause for further concern.”
As might be expected, the local community was unpleasantly surprised by the discovery. A Piketon city councilwoman said, “We aren’t prepared for something like this, that’s for sure… We, at this point, don’t know how far the contamination has reached. That will be part of the ongoing investigation.” She pointed out that nearby bodies of water and homes had tested positive for enriched uranium and neptunium.
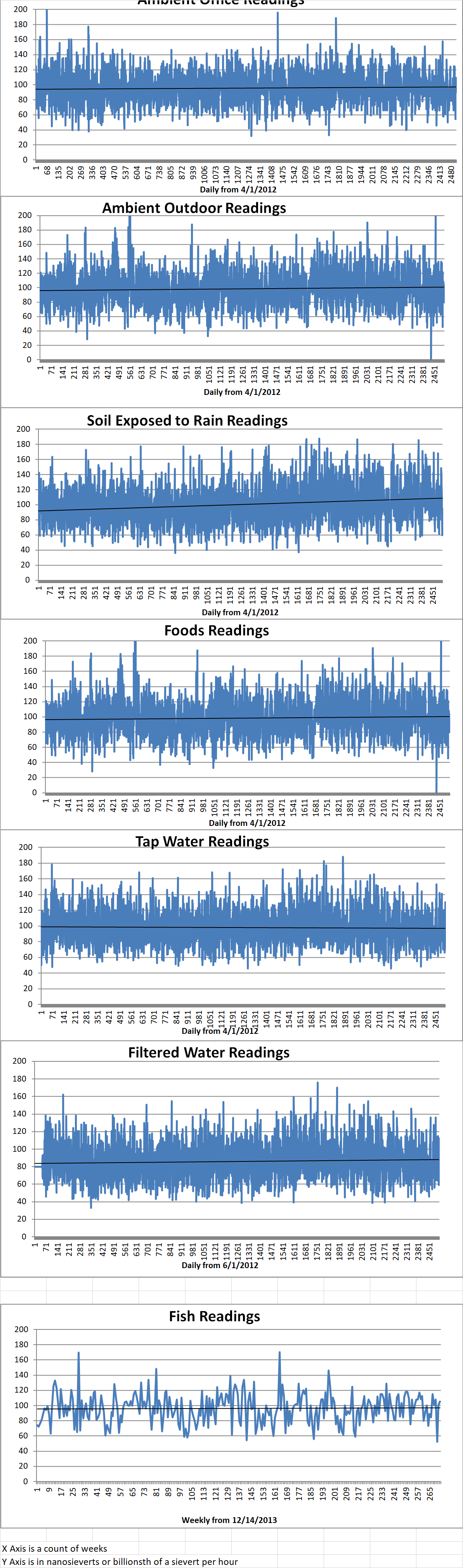
Ambient office = 90 nanosieverts per hour
Ambient outside = 87 nanosieverts per hour
Soil exposed to rain water = 94 nanosieverts per hour
Shallot from Central Market = 95 nanosieverts per hour
Tap water = 130 nanosieverts per hour
Filtered water = 112 nanosieverts per hour