Blog
-
Geiger Readings for May 17, 2014
Ambient office = 90 nanosieverts per hourAmbient outside = 114 nanosieverts per hourSoil exposed to rain water = 134 nanosieverts per hourRaw peanuts from Costco = 77 nanosieverts per hourTap water = 56 nanosieverts per hourFiltered water = 48 nanosieverts per hourHalibut – Caught in Canada = 94 nanosieverts per hour -
New Drug Protects Against Ingestion of Radioactive Particles
I have blogged extensively about the health effects of ionizing radiation. Some organisms have the ability to resist the effects of radiation, primarily through error correcting protein creation. A recent report in the journal Science Translational Medicine details research results of a new method of dealing with radiation damage from the ingestion of radioactive particles which could otherwise be fatal.
A major terrorist threat is the possibility of a “dirty bomb.” This type of bomb does not detonate radioactive isotopes to create a nuclear explosion. Instead, a conventional explosive is surrounded by radioactive materials. When the bomb detonates, radioactive particles are spread over a wide area. The population in the area of the detonation would be vulnerable to inhaling or ingesting these radioactive particles. Another possibility would be to grind up a radioactive material such as plutonium, mix it with the gasoline in the tank of a car and drive the car around a heavily populated area. A tiny amount of plutonium could contaminate a large area and threaten anyone living in that area. The U.S. Institute of Health has been funding research on drugs that could be administered to people living in an area hit by a dirty bomb to help combat the health threat of the dispersed radioactive particles.
A drug named dimethyloxallyl glycine (DMOG) has been tested in mice. This drug neutralizes enzymes which normally function to reduce the level of a particular protein named hypoxia-inducible factor 2. This protein protects the cells that line the digestive tract from radiation damage. With the reduction of the enzymes, higher levels of this protein result in greater protection. In tests, mice given the drug were able to survive a dose of radiation that killed unprotected mice through water loss and diarrhea. Administering “small-molecule dimethyloxallyl glycine (DMOG) increases hypoxia-inducible factor (HIF) expression (responds to reduced oxygen in the cells) , improves epithelial integrity (strengthens the cells lining the gut) , reduces apoptosis (decreases cell death), and increases intestinal angiogenesis (supports formation of new blood vessels) , all of which are essential for radioprotection.” This drug could be distributed to victims of a dirty bomb attack to save lives.
In addition to the possibility use of this drug to treat people exposed to radioactive materials from a dirty bomb, there has also been discussion of the possibility of the use of this drug in radiation therapy. Current radiation therapy for cancer focuses the radiation narrowly on a tumor. With the use of DMOG, it may be possible to subject the whole body of a patient to radiation treatment to deal with a metastasizing cancer which is spreading cancer cells to other parts of the body.
In addition to treating dirty bomb victims and protecting patients receiving radiation therapy, DMOG will be useful in dealing with exposure to radioactive particles loose in the environment from accidents at nuclear power plants and the improper treatment nuclear waste. These will only increase as time goes by and the use of such drugs may eventually be widespread.
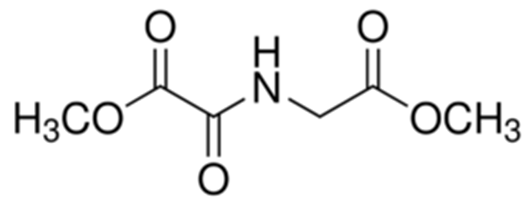
Structure of dimethyloxallyl glycine:
-
Radiation News Roundup May 16, 2014
A new study reveals deaths and mutations ”increased sharply” from exposure to Fukushima contamination, “especially at low doses.” enenews.com
Animals dead from “mystery disease” on the Pacific coast of Alaska are being tested for Fukushima radiation. enenews.com
Wildfires had burned nearly 1,000 acres in San Diego County by Thursday afternoon but did not pose a threat to the San Onofre Nuclear plant shuttered last year. nuclearstreet.com
-
Geiger Readings for May 16, 2014
Ambient office = 103 nanosieverts per hourAmbient outside = 65 nanosieverts per hourSoil exposed to rain water = 76 nanosieverts per hourRaw peanuts from Costco = 127 nanosieverts per hourTap water = 95 nanosieverts per hourFiltered water = 87 nanosieverts per hour -
Nuclear Reactors 126 – Risk Factors and the Retirement of U.S. Nuclear Power Reactors
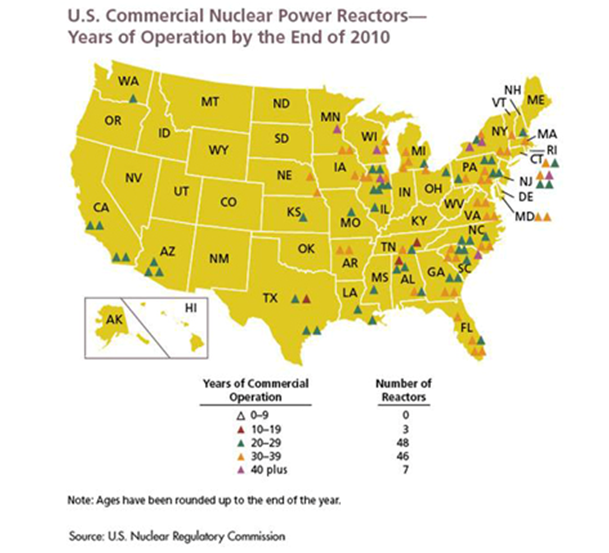
I have blogged a lot about the decline of the U.S. nuclear industry. The Fukushima disaster, rising costs, aging reactors, cheap natural gas and other factors have been eroding support and investment for new reactors. Recently a report, Renaissance in Reverse: Competition Pushes Aging U.S. Nuclear Reactors to the Brink of Economic Abandonment, came out and offered some details about the future of the nuclear industry in the United States. A few years ago, there were one hundred and four nuclear power reactors in the United States. Four of those reactors have been designated for permanent closure. Some of the closures were related to technical issues but one happened because the operators could not make a profit and could not find a buyer. Another reactor was shut down because it had become too expensive to fix. In addition there have been cancellations of five projects to increase the output of five reactors because of rising costs.
The report utilizes eleven risk factors to project the future of individual reactors. including cost of electricity greater than the current wholesale market, commissioned before 1974, smaller than seven hundred megawatts, major systems broken, suffering multiple outages, single reactor at power plant, suffering long term outages, multiple safety issues and needing retrofit because of new standards triggered by Fukushima disaster. These risk factors were extracted from Wall Street analyses that were intended for investors who might be considering an investment in nuclear power.
When the hundred operating reactors are tested against these risk factors, over three dozen of the operating U.S. power reactors have four or more of the risk factors. The purpose of this report is to indicate which of the existing reactors are most likely going to be retired soon. A review of the report reveals that many of the problems that suggest retirement are not new but have been around since the beginning of nuclear power generation in the U.S. “The problems are endemic to the technology and the sector.” In addition, the rising cost of keep the aging U.S reactor fleet working and the presence of low cost alternative energy sources including cheap natural gas and renewables will likely continue for the next several decades.
When I criticize nuclear power, proponents offer a number of defenses, some of them sound and some questionable. I always patiently explain that nuclear power is an extremely complex subject with economic issues, health issues, political issues, social issues, environmental issues, technical issues and other considerations. While it might be defendable on some specific challenges, when many factors are taken into account, it becomes obvious that nuclear power is just not a viable choice for the future of U.S. energy generation. The analysis in the report goes a long way toward validating my contention. As the Wall Street Journal analysis found, investing in the replacement of old U.S. power reactors with new nuclear reactors is just not a good bet.
-
Radiation News Roundup May 15, 2014
Work has begun towards implementing a way to remove the melted fuel at Fukushima Daiichi. fukuleaks.org
In the United Kingdom, Sellafield Ltd is pressing cutting-edge 3D printing technology into service to help meet the challenges of decommissioning one of the world’s oldest and most complex nuclear sites. world-nuclear-news.org
-
Geiger Readings for May 15, 2014
Ambient office = 125 nanosieverts per hourAmbient outside = 104 nanosieverts per hourSoil exposed to rain water = 95 nanosieverts per hourRaw pistachio from Top Foods = 104 nanosieverts per hourTap water = 88 nanosieverts per hourFiltered water = 77 nanosieverts per hour -
Radioactive Waste 77 – Update on the Recent Accident at the Waste Isolation Pilot Plant 3
I have recently been posting about the undetermined release of radioactive materials from the Waste Isolation Pilot Project near Carlsbad, New Mexico. In February, there was an event that released plutonium and americium into the atmosphere. Despite months of investigation, the ultimate cause of the radiation release still has not been determined. The WIPP is the only repository for low level waste, tools, clothing, etc. generated by the production of nuclear weapons for the U.S. arsenal. It is located in an old salt mine. Since the radiation release, the WIPP has been closed and there are estimations that it may remain closed for up to three years.
A new theory has been proposed to explain the radiation release. The waste shipped to the WIPP is contained in sealed drums. WIPP will not accept any liquid waste so some substance has to be added to any drum containing liquid to soak it up. In the past, the substance added was an inorganic clay-based absorbent similar to commercial cat litter. Such absorbents have been used to soak up chemical spill for many years. In the nuclear industry, these clay absorbents were routinely used to soak up liquids that were used to clean laboratories. The silicate minerals in the clay bound to and stabilized ammonia nitrates in the waste water.
Recently, a change was made to an organic absorbent made from wheat which is also used in commercial cat litter. It has been suggested that the new organic absorbent may have caused a chemical reaction that allowed nitrate salts to dry out instead of being bound and stabilized. These unstable nitrate salts could have caused a “mild” explosion that broke open some of the drums and released the radioactive isotopes. There is some evidence of melted seals on some drums of waste which suggests that something in or near the drums generated heat.
Inspectors of the sealed drums would not have seen anything that would have alerted them to the problem. The testing that is done for the buildup of gases above the materials in the drums before they are stored would also have given no indication of the problem.
There are drums that contain the new absorbent in the WIPP and more are piling up at temporary locations until the cause of the February radiation release has been determined. Environmental experts demand that those drums which are outside of the WIPP be moved inside and that drums in temporary storage be shipped to the WIPP. If the room is properly sealed as soon as the drums are stored, that should prevent a future radiation release. If the drums in temporary storage are not moved to the WIPP and sealed in then there may be more explosions in less secure places, releasing much more radiation into the environment.
The experts think that the absorbent explanation is the most likely cause of the radiation release but are not completely sure. In any case, this situation is illustrative of how complex nuclear technology can be. Apparently no one really investigated what organic absorbent could do before putting it into the drums. How many other bad decisions based on incomplete information will result in dangerous accidents?
Drums of low-level waste diverted from the WIPP to temporary storage in Texas:
-
Radiation News Roundup May 14, 2014
Hundreds fall ill after Fukushima nuclear plant rubble is burned in a major Japanese city. enenews.com
Tons of ‘highly radioactive’ liquid are pouring out of the Fukushima Unit 2 Reactor each day. enenews.com
China’s central government announced Monday that it is forming a fast-response team to assist in emergencies at civilian nuclear facilities. nuclearstreet.com