There are many arguments against the use of nuclear reactors to generate electrical power. A few of the major objections are listed below.
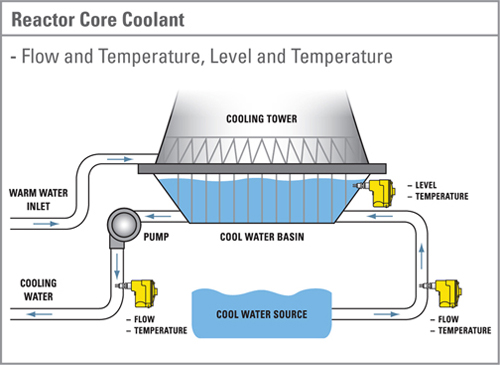
- Risk of accidents. If everything and everyone at a nuclear plant work perfectly, nuclear power is a safe source of electricity. Unfortunately, we don’t live in a perfect world. Most of the worlds reactors are being run safely but there have been many minor and major problems with equipment, oversight, human errors, reporting, leaks at many nuclear power plants. Fortunately most of these did not result in serious threats to the environment or human life but there were a lot of close calls.
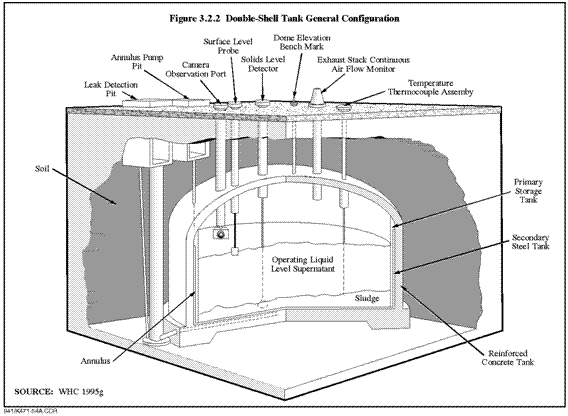
- Lack of a permanent solution to accumulating nuclear waste. A number of different techniques and locations have been proposed for the permanent safe storage of nuclear waste. None of these have been implemented. An expansion of nuclear power would add to the existing body of nuclear waste.
- Lack of agreement over available world supplies of uranium. Uranium is a common element and there are many different deposits of various grades of uranium ore. However, there has been no agreement on how much uranium is available to fuel the worlds reactors. Estimates based on complex variable range from only 25 years worth to hundreds of years of potential fuel.
- Nuclear energy is not as cheap as has been claimed. Costs of transport and storage of fuel and waste as well as insurance to cover accidents are not usually included in the costing of nuclear power. Governments are using tax dollars to pay for these services to the nuclear industry.
- Nuclear energy is slowing a transition to renewable energy sources. As long as the idea of a massive plant burning expensive fuel is attractive to energy suppliers, there will be a lack of urgency in transitioning to alternative energy sources. There is a real competition for dollars, subsidies and tax breaks between nuclear and renewable energy sources.
- Liability of nuclear power plant operators is limited for accidents. In the United States and other nuclear countries, there are caps on the total amount of payments an operator will have to make in case of a major accident. All responsible experts agree that these caps are far too low to cover the cost of clean-up of a disaster like Fukushima. Taxpayers would be on the hook for the additional costs.
- Nuclear power produces carbon dioxide. While the actual operation of a nuclear reactor produces very little carbon dioxide, When considering the whole life cycle of mining, refining, plant construction, fuel burning, waste storage and disposal and power plant decommissioning, nuclear power does add to human carbon dioxide generation above the level of renewable.
- Lack of sufficient graduates in nuclear science. Due to the negative press of nuclear power and the lack of new plants being built, universities are not graduating enough nuclear engineers to design, build and operate future reactors.
- Nuclear power can pave the way for nuclear weapons. Development of peaceful nuclear power generation can provided equipment and expertise that can be turned to the production of nuclear weapons. The world needs fewer nuclear weapons, not more.
No Nukes sign from Sodahead.com: