The pressurized water reactor (PWR) is the most widely used design in nuclear reactors for electrical power generation in the world. The PWR was originally intended for use as a propulsion system for nuclear submarines. It was used in the second commercial power plant in the United States. Since then it has seen widespread use in the United States and other countries such as France for power generation. The PWR reactors currently in use in the United States are referred to as Generation II reactors.
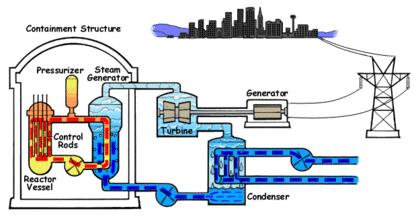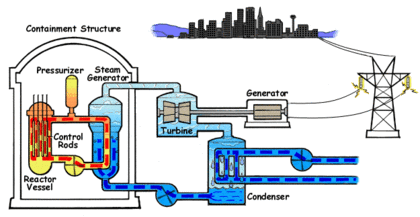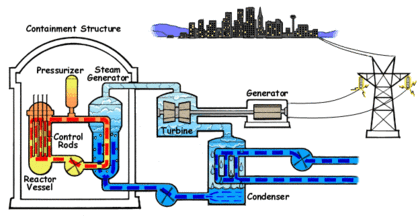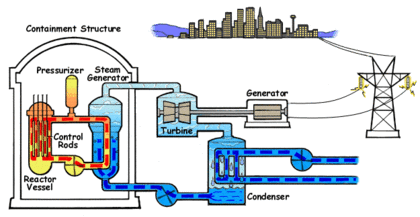
In the PWR reactor ordinary or light water is used as the coolant. The water that circulates through the reactor core to capture the heat of the fission reaction is kept under high pressure and does not turn into steam despite the high temperature. The water from the core is passed to a steam generator where its heat is transferred to a separate water circulation system where steam is created.
In a power generation plant, this steam is used to spin a turbine which generates electricity. A third water system passes water through a condenser which cools the steam and turns it back into liquid water. This third system requires large amounts of water which is drawn from and sent back to a lake, river or ocean at a higher temperature.
In propulsion systems, the steam can be fed through a system of gears to a propulsion shaft which turns propeller blades. The expansion of steam can be used in a piston system to drive a catapult to launch aircraft. In some applications, the heat from the steam can be directly extracted for use in industrial processes.
The water in PWRs also acts as a moderator for the fission reaction in the core. As the fast neutrons generated by fission collide with water molecules, they are slowed down which increases their ability to trigger more fission events in the core. As the water heats, the molecules move apart and reduce the ability of the water to slow down the fast neutrons. This in turn reduces the activation of fission events which cools the core. This creates a negative feedback control system which keeps the core within a particular temperature range. The position of the control rods determines the center or set point of the temperature range. This system is an important safety feature in PWRs. Light water is an excellent moderator and allows the construction of compact reactor cores.
The fuel used in PWRs is uranium dioxide (UO2) ceramic pellets in Zircaloy fuel rods. Between two hundred and three hundred fuel rods will be used in a typical reactor. They are arranged in a square configuration with a hole in the center for a control rod. One hundred and fifty to two hundred and fifty rods are assembled into the reactor core. Usually every eighteen to twenty four months, one third of the rods will be replaced.
Boron control rods are used in PWRs to start the reactor, shut down the reactor and to control short terms changes in power demand. The control rods can also compensate for changes in the fuel due to depletion or poisoning by isotopes generated during fission. Boric acid is mixed with the coolant water to change the neutron absorption rate.
PWRs are very stable and the separate coolant and steam systems prevent radioactive contamination of the steam. If offsite power is lost, the electromagnets holding the control rods shut off and the rods are inserted completely into the core by gravity which results in automatic shutdown. This automatic shut down is an important safety feature, however the radioactive decay in the fuel still proceeds at a low rate and requires up to three years of continuous coolant circulation in order to prevent overheating and the possibility of a meltdown. The high pressure required in a PWR demands very strong piping and a heavy reactor vessel. Neutron flux makes the metal in PWRs brittle over time and limits the lifespan of the reactor.