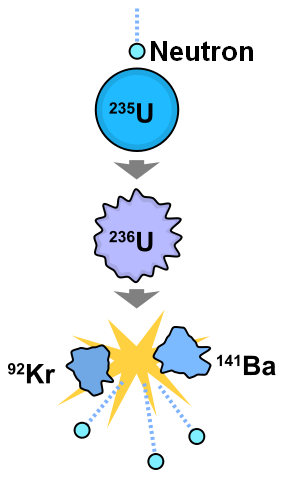
When uranium metal is processed to increase the proportion of U-235, a byproduct of the process is a great deal of uranium metal containing smaller amounts of U-235 than the natural proportion of 0.72 %. This byproduct is known as “depleted” uranium(DU). The U-238 in DU emits alpha particles which contain 2 protons and 2 neutrons. These alpha particles only travel a few centimeters in open air and can be blocked by a sheet of paper or plastic, a layer of clothing.
DU can enter the human body through in breathing, drinking, eating or skin contact and penetration by fragments of uranium munitions. The exact chemical properties of the uranium, the amount of the contamination, the way it enters the body and other factors determine its effect on health. The uranium is absorbed by tissue and distributed by diffusion, blood circulation, air passages and digestive tract and eventually excreted out of the body. All of these processes have their own impact on how the depleted uranium may damage the exposed individual.
In addition to being mildly radioactive, DU is a toxic heavy metal although not as toxic as mercury. The danger from toxicity is about a million times greater than the danger from radioactivity. DU causes damage to the kidneys as it is eliminated from the body. It can pass the blood-brain barrier and accumulate in the different parts of the brain to cause neurological problems. DU causes problems with bone formation and can inhibit wound healing. The lungs are especially vulnerable to damage by the toxicity of DU with lesions, fibrosis, edema, swelling and hemorrhaging. There can be conjunctivitis, inflammation and ulceration of the eyes from DU. Red blood cell count and hemoglobin can be reduced in the blood.. If pregnant women are exposed, there can be birth defects in the child. The radioactivity of DU can cause lung, lymph and brain cancer.
The use of DU in munitions has resulted in serious contamination in theaters of war such as Iraq and Afghanistan. When the munitions explode or impact, huge amounts of DU dust are spread over wide areas and then inhaled by soldiers and civilians. There is no effective way to clean up such wide spread contamination. The zone of contamination will spread as wind storms pick up the dust and distribute it more widely. Rain will wash it down streams and rivers into lakes and the sea.
Since the first Gulf War in 1991, millions of Iraqis have been exposed to higher levels of radiation that normal background radiation in the natural environment from the hundreds of tons of DU munitions used. Since 1995, there has been a documented increase in the number of cases low level radiation exposure related diseases such as leukemia, birth defects, breast cancer and other illnesses in Iraq. The age of leukemia victims has been declining and the higher incidence levels can be correlated with the area with higher concentrations of DU. Gulf War Syndrome may be related to exposure of soldiers to DU.
There have been charges that the Pentagon has deliberately concealed the amount of DU munitions used in Iraq and Afghanistan, downplayed the extent of the contaminated areas, and dismissed health concerns of the Iraqi people and returning veterans. Various countries and NGOs have made repeated calls for a ban on the use of DU in munitions due to these serious concerns about the health effects of DU dust in theaters of war.