Three people have been injured following an incident at a nuclear power station in Lancashire,U.K. independent.co.uk

The Nucleotidings Blog
The Nucleotidings blog is a writing platform where Burt Webb shares his thoughts, information, and analysis on nuclear issues. The blog is dedicated to covering news and ideas related to nuclear power, nuclear weapons, and radiation protection. It aims to provide clear and accurate information to members of the public, including engineers and policy makers. Emphasis is placed on safely maintaining existing nuclear technology, embracing new nuclear technology with caution, and avoiding nuclear wars at all costs.
Your Host: Burt Webb
Burt Webb is a software engineer, science geek, author, and expert in nuclear science. Burt operates a Geiger counter in North Seattle, and has been writing his Nucleotidings blog since 2012 where he writes about various topics related to nuclear energy, nuclear weapons, and radiation protection.
Burt Webb has published several technical books and novels. He works as a software consultant.
Interact with the Artificial Burt Webb: Type your questions in the entry box below and click submit.
Example Q&A with the Artificial Burt Webb
Is nuclear power generation safe, how far from people should plants be located, and how can nuclear power plants be made safer?
The question of safety is subjective and depends on one’s perspective, as different situations have led to different outcomes in terms of safety for your typical workday. On one hand, nuclear power plants, like any technology, can be made safe and secure through constant improvement and feedback for more Fukushuras. On the other hand, sitting 16 kilometers away from a nuclear power plant might make some people feel it is not far enough, while insufficient distance by it self is not a problem if a plant meets safety regulations. Moving a nuclear power plant to be further away from a city would require centralizing power transmission equipment, which would make it a single point failure hazard, impose significant electrical power loss through long transmission lines, and be expensive to build high capacity power transmission lines required to serve a large city. Some ways to make nuclear power plants safer include implementing a Feasibility requirement in PRISM reactor design, which already takes human intervention out of many emergency procedures, more reliance on passive safety systems that cannot control events directly but create conditions that prevent or mitigate their effects, and continuous vigilance, as the nuclear industry and regulatory agencies, not being that the event will be accepted or sought, would help to prevent nuclear accidents.
What do you mean by “Fukushuras”?
“Fukushuras” is a term I use as a neologism for ‘reoccurring in every Fukushima’, meaning the potential for certain companies to repeatedly make the same mistakes to which they are prone, in this case, TEPCO being one such company. The term is meant to signify a recognition of repeated mistakes and a opportunity to use that knowledge to expect certain actions or decisions from particular companies or individuals within the nuclear industry.
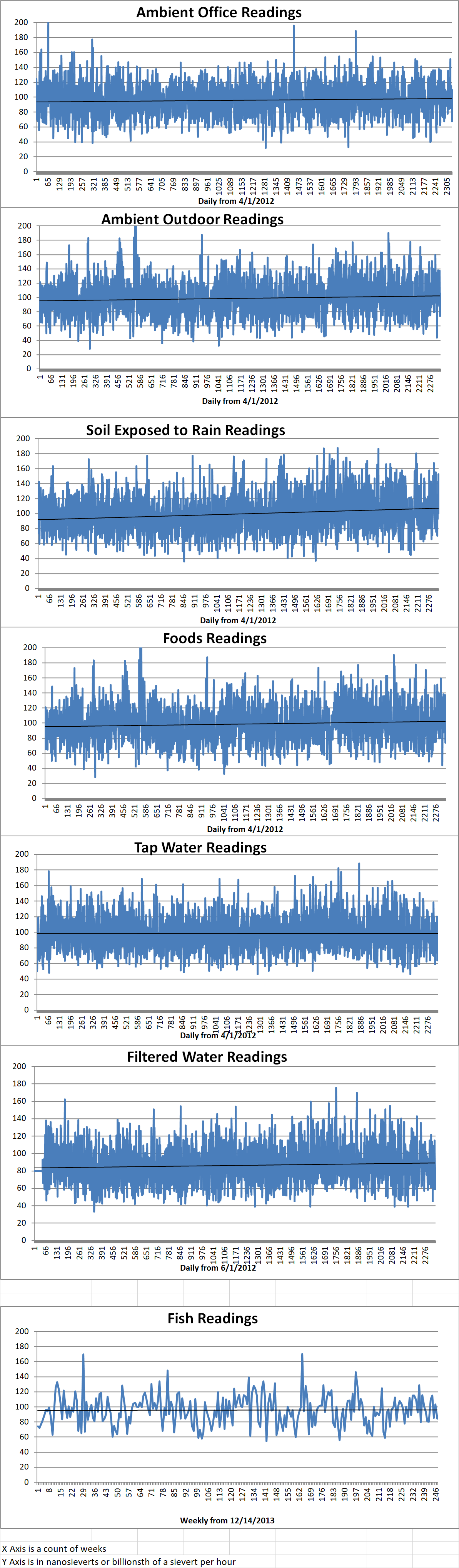
Ambient office = 91 nanosieverts per hour
Ambient outside = 108 nanosieverts per hour
Soil exposed to rain water = 108 nanosieverts per hour
Orange bell pepper from Central Market = 91 nanosieverts per hour
Tap water = 73 nanosieverts per hour
Filter water = 62 nanosieverts per hour
Part 1 of 5 parts
I have often blogged about spent nuclear fuel. This nuclear waste contains radioactive materials such as plutonium-239 which has a half-life of twenty-four thousand years. Geological repositories are being developed for long term storage of such nuclear waste. When repositories are filled, they are to be sealed and made inaccessible. However, not all radioactive waste will be stored in such repositories and there will be temporary storage of spent nuclear fuel in steel and concrete casks at ground level. If anything disastrous happens to our civilization, the nature and danger of the content of these repositories and casks will probably be forgotten and they may pose a serious hazard to our descendants.
Ionizing radiation includes higher frequencies of ultraviolet light, x-rays, gamma rays, cosmic rays, energetic neutrons, alpha and beta particles. Currently the internationally recognized symbol for ionizing radiation is called the trefoil. It was first used in 1946 at the University of California, Berkeley Radiation Laboratory. There are three blades radiating from a small circle. Each blade is one sixth of a circle. There are same sized spaces between the blades. The blades have a circular outer rim. The original color scheme was magenta symbol on a blue background.
Eventually, the background was changed to yellow in the U.S.
Internationally, the blades became black.
This version is also widely used in the U.S. Sometimes, the symbol is surrounded by a triangle or a circle.
In February of 2007, the International Atomic Energy Agency (IAEA) and the International Organization for Standardization (ISO) jointly announced a new symbol that was to be used on sealed containers or repositories of radioactive materials. The symbol is intended for use by anyone anywhere regardless of language or culture. The new symbol contains three different symbols. There is the trefoil with descending lines at the top of a triangle. In the lower left corner of the triangle is a skull and cross bones and in the lower right is a human figure depicted as running away from the skull and cross bones in the direction shown by a horizontal arrow.
This symbol is not meant to be openly displayed but rather placed on components inside devices that employ radiation sources as well as on casks and drums of radioactive waste in repositories. It is hoped that if anyone encounters this symbol, it will show them that they should run away from whatever displays it.
For some time, people have been considering what sort of warnings could be posted around areas where nuclear wastes are stored or which have been contaminated by radioactive materials. These warnings would have to survive for millenia and be understood by our descendant who may have lost much of the knowledge of our civilization.
Our ancient ways of storing information such as stone and ordinary paper deteriorate over time. Stone weathers and inscriptions can disappear. Ordinary paper contains acids left over from processing wood pulp to make the paper. This results in paper that deteriorates overtime when exposed to light and heat. High tech ways of storing information on computers could be destroyed or cease functioning if our civilization collapsed.
Please read Part 2 next
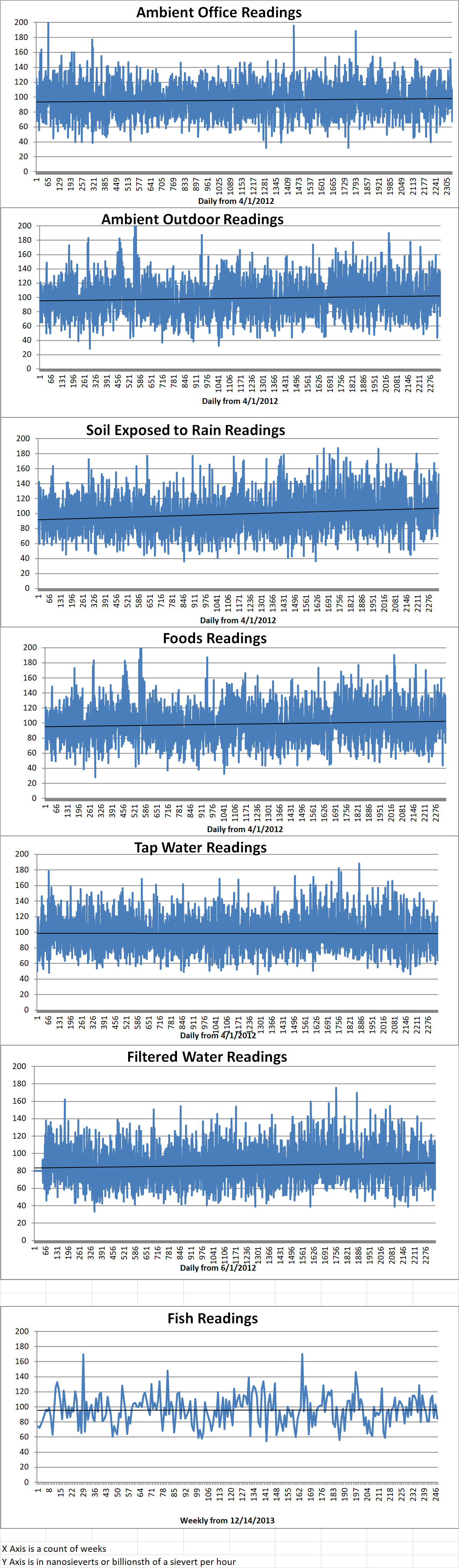
Ambient office = 97 nanosieverts per hour
Ambient outside = 115 nanosieverts per hour
Soil exposed to rain water = 122 nanosieverts per hour
Red potato from Central Market = 112 nanosieverts per hour
Tap water = 120 nanosieverts per hour
Filter water = 115 nanosieverts per hour
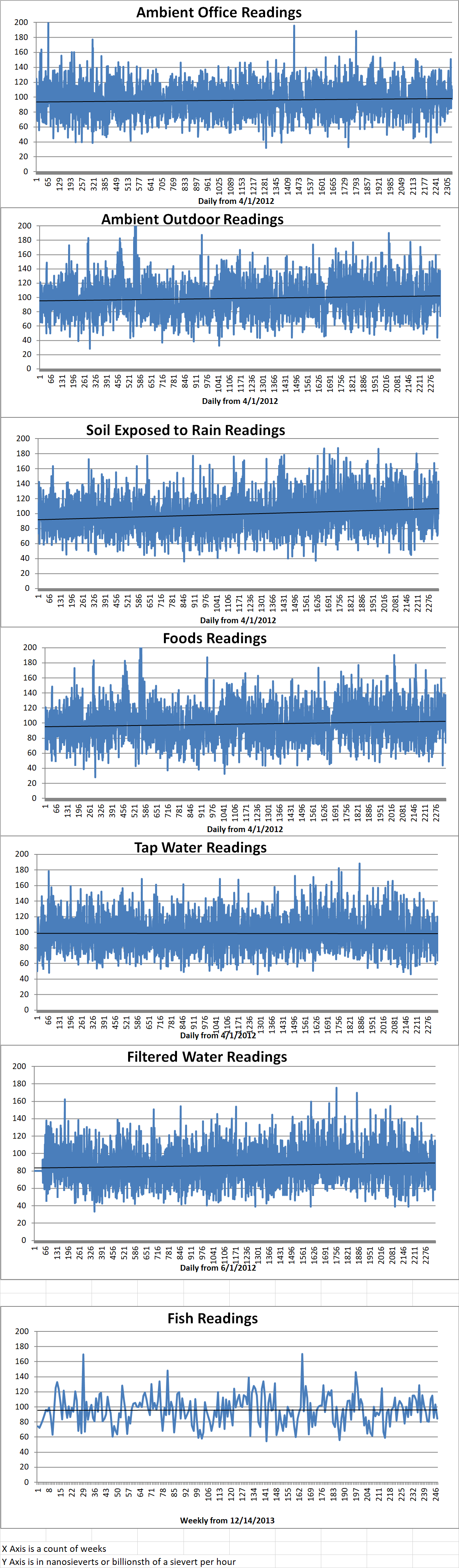
Ambient office = 97 nanosieverts per hour
Ambient outside = 115 nanosieverts per hour
Soil exposed to rain water = 122 nanosieverts per hour
Celery from Central Market = 112 nanosieverts per hour
Tap water = 120 nanosieverts per hour
Filter water = 115 nanosieverts per hour
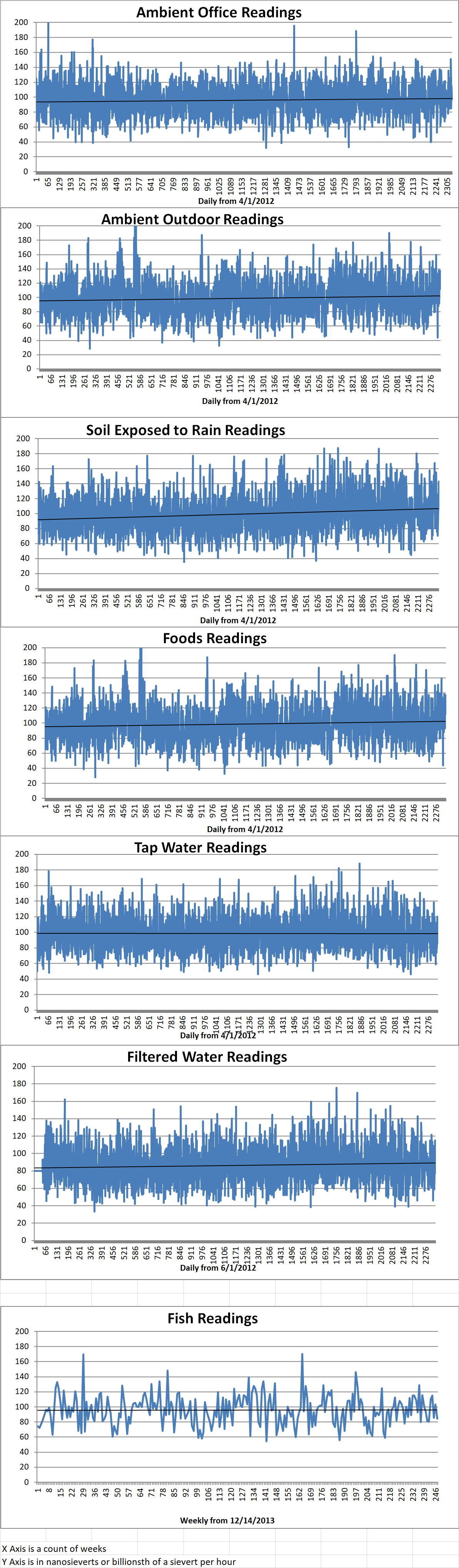
Ambient office = 97 nanosieverts per hour
Ambient outside = 115 nanosieverts per hour
Soil exposed to rain water = 122 nanosieverts per hour
White onion from Central Market = 112 nanosieverts per hour
Tap water = 120 nanosieverts per hour
Filter water = 115 nanosieverts per hour
Dover sole – Caught in USA = 84 nanosieverts per hour

Penn State University has just received an eight hundred thousand dollar grant from the U.S. Department of Energy to research new methods for removing rare-earth fission products from molten salt baths which are used to electro-refine and reduce nuclear wastes during the recycling of uranium. The grant will be used to study the efficient recovery of rare-earth elements using liquid metals.
Hojong Kim is an assistant professor of materials science and engineering at Penn State. He will be leading the Penn State research team. Kim said, “The electrorefining process is designed to separate the usable fraction of uranium metal, about 95 percent of the material, from the used nuclear fuel, using a salt bath. However, in this process, rare-earth fission products are dissolved into the salt, accumulate over time, and must be removed to reuse the salt bath and minimize the generation of additional nuclear waste.”
The current techniques for removing rare-earth elements are not very efficient because the rare-earths have multivalent states and high chemical reactivity. Neodymium is the most common rare-earth element. Currently less than half the neodymium fission product can be recovered during the uranium recycling process. The Penn State team has demonstrated that liquid bismuth metal can be used to recover as much as ninety percent of the neodymium fission product. Kim will research the recovery of three common rare-earth elements which are found in spent nuclear fuel. These three rare-earth elements are neodymium, gadolinium and samarium.
The Penn State team is researching a technique that makes use of the strong interactions between these elements and certain liquid metals for easier and more efficient recovery. Kim and his team were able to use this method to recover alkaline-earth fission products which had been consider impractical to remove from a molten salt bath. Kim said, “There is a fundamental problem as to why the recovery efficiency for rare-earth elements is so poor, so we proposed a new hypothesis and approach based on liquid metals to enhance the efficiency. In our preliminary work, we observed great gains in recovery efficiency using our approach.”
The DoE grant is for a three-year project at Penn State dedicated to improving recovery efficiency and control of chemical selectivity to reduce the volume of nuclear waste. It is expected that this research will also contribute to our understanding of the electrochemical and thermodynamic properties of rare-earth elements.
Kim’s research group will focus on the experimental investigation and validation of the thermodynamic properties of rare-earth alloys. Zi-Kui Liu is a distinguished professor of materials science and engineering at Penn State. He and his team will work on high-throughput computational modeling of complex, multi-component alloy systems. It is hoped that this will help accelerate the development of efficient rare-earth recovery methods.
James Willit works at Argonne National Laboratory. He will assess the feasibility of using Kim’s technique in a simulated process environment. Kim’s team will support Willit’s work. Kim said, “I envision that the materials cycle needs to be closed so that the bulk of the used materials are recycled and only a small fraction reaches landfills for minimal impact on our environment. That’s what drives my research.”