“Should the U.S. bail out failing nuclear and wind energy producers?” tri-cityherald.com
Blog
-
Geiger Readings for Aug 10, 2018
Ambient office = 98 nanosieverts per hour
Ambient outside = 154 nanosieverts per hour
Soil exposed to rain water = 153 nanosieverts per hour
Crimini mushrooms from Central Market = 137 nanosieverts per hour
Tap water = 91 nanosieverts per hour
Filter water = 80 nanosieverts per hour
-
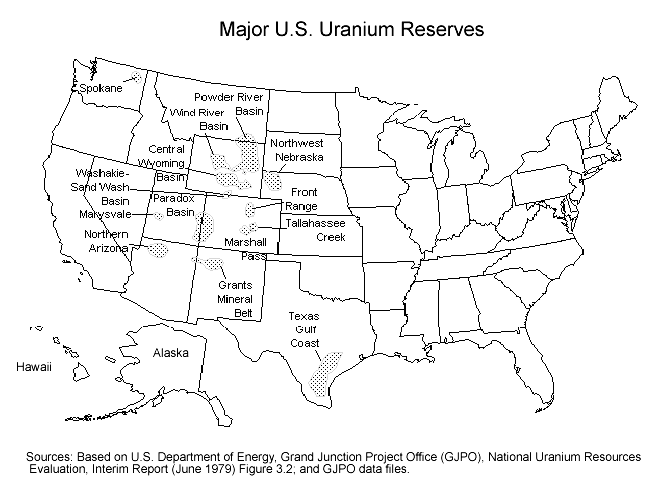
Nuclear Reactors 600 – History Of Uranium Production In The U.S. – 1 of 4 Parts
Part 1 of 4 parts
I have blogged before about uranium mining and the global uranium marketplace before. Today I thought I would dive more deeply into the uranium mining industry in the U.S.
The U.S. uranium mining industry began around 1955 and flourished during the Cold War until about 1980. The U.S. had generous incentives for uranium mining because it was building up its nuclear arsenal during the Cold War. A uranium market analyst said, “It’s been government-sponsored, government-subsidized just since the beginning. Trying to sort that out and find where there’s a free market in uranium — I find that very questionable.”
The U.S. incentives included a ten -ear guarantee for the price that the federal government would pay for high-grade uranium ore. There was also a ten-thousand-dollar discovery and production bonus for each new ore deposit that was opened. Factoring in inflation since then, the bonus would be almost a hundred thousand dollars in today’s currency. These incentives triggered a uranium “gold rush” in the western states. As one analysist said, “It was crazy around this part of the country. Everyone with a jeep and a Geiger counter was out trying to get rich.”
During the 1960s, the federal government had filled warehouses with enough uranium stockpiles to the extent that the incentive program was not longer needed, and it was cancelled. However, there were rules that stayed in place against the import of foreign uranium until 1975. After that a growing percentage of foreign uranium entered the marketplace.
Inexpensive high-quality uranium ore came from deposits in Canada and Australia. By 1987, the U.S. was importing almost fifteen million pounds of uranium. Domestic production fell by a third to about thirteen million pounds. Although foreign competition did affect U.S. uranium production, the construction boom of commercial power reactors in the 1970s did keep U.S. mines in operation
The meltdown of a reactor at Three Mile Island in 1979 triggered a powerful backlash against nuclear power in the U.S. In 1986, the nuclear disaster at Cernobyl in Ukraine irradiated the country side and caused the permanent evacuation of a Ukrainian city. The U.S. love affair with nuclear power ground to a halt.
During this period, utilities were becoming concerned about the time and money necessary to license and construct nuclear power plants. Actual construction of nuclear power plants fell well below projections for the 1980s. This, in turn, depressed the market for uranium. When the last wave of nuclear power plants came on line in 1990, U.S. uranium production was at a thirty-
five year low.
Following the fall of the Soviet Union in 1991, it was revealed that the Soviets had been dumping cheap uranium ore onto the U.S. market prior to the breakup. The U.S. put restriction on the import of uranium ore from the old Soviet bloc countries in 1992 which provided some temporary relief for U.S. uranium miners. However, in 1993, the U.S, signed a contract for the Megatons to Megawatts program with Russia to buy five hundred tons of weapons-grade uranium. The high-grade uranium would be mixed with low grade ore to reduce the purity so that it could be made into nuclear fuel for U.S. power reactors. Over the next twenty years, this Russian uranium was used to supply about one third of the fuel for U.S. commercial nuclear power plants.
Please read Part 2 -
Geiger Readings for Aug 9, 2018
Ambient office =100 nanosieverts per hour
Ambient outside = 153 nanosieverts per hour
Soil exposed to rain water = 152 nanosieverts per hour
Carrot from Central Market = 118 nanosieverts per hour
Tap water = 96 nanosieverts per hour
Filter water = 80 nanosieverts per hour
-

Nuclear Reactors 599 – NRC Is Proposing New Looser Rules For Decommission Nuclear Power Reactors
The Nuclear Regulatory Commission has released a regulatory analysis of the regulatory basis supporting a “rulemaking” to change the NRC’s decommissioning of nuclear power reactors. The NRC has multiple goals for changes to the current regulations. First, they want to make the decommissioning process more efficient. Second, they want to reduce the need for granting exemptions to existing regulations. Third, they are going to consider other decommissioning issues that are identified as relevant by the NRC staff. Fourth, they want to support the principles of good regulation, including openness, clarity, and reliability.
The NRC is recommending rule making in the areas of “emergency planning, physical security, cyber security, drug and alcohol testing, training requirements for certified fuel handlers, decommissioning trust funds, applicability of backfitting provisions, and offsite and onsite financial protection requirements and indemnity agreements.”
The revised regulations would “formalize steps to transition a power reactor from operating status to decommissioning while reducing the need for exemptions and license amendments.” There are also recommendations to clarify requirements regarding topics such as spent fuel management and environmental reporting.
In addition to the recommendations for rule making, there are also updates for guidance that deals with aging management, the proper roles for State and local governments in the decommissioning process, the level of NRC review of a Post-Shutdown Decommissioning Activities Report, the options that exist for decommissioning and the schedule for decommissioning.
The regulatory analysis was carried out in order to assess the economic impact on the nuclear industry, government and society that would be the result of the rule making and guidance contemplated by the NRC. The NRC staff laid out the various decommissioning alternatives and discussed the cost benefit analysis. The conclusion is that the staff recommendations for rule making and guidance development is ultimately cost beneficial to the nuclear industry, government and society.
Four Democratic sent a letter to the NRC warning that the new rulemaking and guidance updates could pose a threat to safety, security and emergency preparedness with respect to decommissioning nuclear power plants.
Senator Ed Markey, a member of the Environment and Public Works Committee and one of the four authors of the letter, said “We need a decommissioning rule that acts as a plan for addressing the myriad difficult issues, including the challenges posed by climate change like rising sea levels, that communities will face as nuclear power plants across the country prepare to shut down. The challenge of decommissioning Pilgrim Nuclear Power Plant in a safe and expeditious manner would only be greater because of this draft rule.”
“This is a missed opportunity to put down a marker for smarter decommissioning, and I urge the Commission to strengthen this draft rule to ensure safety not expediency is paramount. I will be following up with the Nuclear Regulatory Commission to understand why key provisions requested by the public are missing from this proposed decommissioning rule.”
Earlier this year, Senators Ed Markley, Kamala D. Harris, Bernie Sanders and Kirsten Gillibrand co-sponsored legislation to “…improve the safety and security of decommissioning reactors and the storage of spent nuclear fuel at nuclear plants across the nation.” Markley has also introduced the Dry Cask Storage Act. This legislation would require that every nuclear reactor operator comply with an NRC-approve plan that requires that the spent nuclear fuel at a decommissioned plant be safely removed and placed in dry casks storage within seven years of the time that the decommissioning plan was submitted to the NRC. -
Geiger Readings for Aug 8, 2018
Ambient office = 106 nanosieverts per hour
Ambient outside = 87 nanosieverts per hour
Soil exposed to rain water = 87 nanosieverts per hour
Orange bell pepper from Central Market = 129 nanosieverts per hour
Tap water = 82 nanosieverts per hour
Filter water = 73 nanosieverts per hour
-
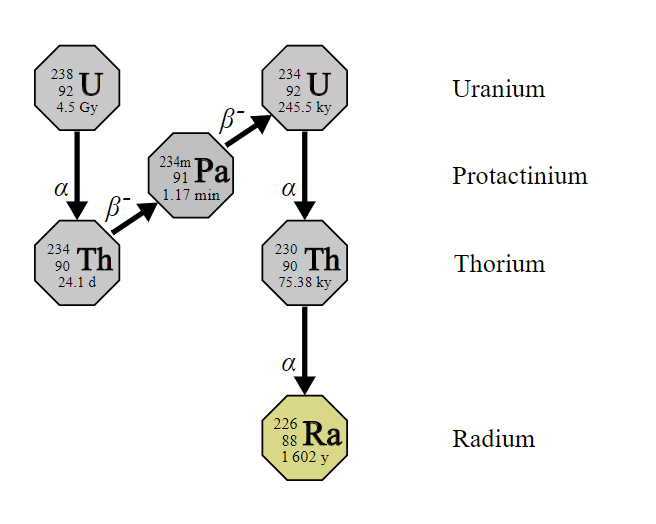
New Production Process For Radium Isotopes Is Being Developed
Radioactive isotopes are used in many medical diagnostic and treatment procedures. In recent years, there have been problems with the production and distribution of radioisotopes used to treat cancer. New technologies are being developed for the production of medical radioisotopes. Now a new process for producing pure radium for cancer treatment is under development.
Radium is a chemical element with the symbol Ra and an atomic number of eighty-eight. It is an alkaline earth metal. All the isotopes of radium are highly radioactive with the most stable isotope being radium-226. It has a half-life sixteen hundred years and it decays into radon-226 gas. Radium is found in trace amounts in natural deposits of uranium and thorium. It is not a necessary element for living systems and can damage health when incorporated into biochemical processes. The only commercial use for radium is in nuclear medicine. The global production of radium is about five pounds per year.
Radium is a radioelement that tends to wind up in the bones of living creatures. The most important element in the bone mineral hydroxyapatite is calcium. Radium is similar to calcium, so it can replace the calcium in hydroxyapatite. It accumulates in rapidly proliferating cancel cells in bone metastases. Once it has been incorporated into the cancer cells, the alpha particles emitted by the radium cause the death of the cancer cells.
Radium-223 was the first alpha emitting isotope that was approved by the Food and Drug Administration for the treatment of cancer. Two other radium isotopes, radium-224 and radium-225, are used in preclinical research. The researchers at Los Alamos created a new method for automatically recovering such radioisotopes from targets of irradiated thorium.
There are huge deposits of thorium around the world which are largely unexploited. There has been research into thorium as a possible nuclear fuel since the 1950 but there are no operational thorium power reactors in the world. However, this may change because thorium research has been accelerating lately, especially in India which has huge deposits of thorium.
Researchers from the Los Alamos National Laboratory’s Isotope Team worked with collaborators from Brookhaven National Laboratory and Oak Ridge National Laboratory to develop a new industrial process for creating pure radium. The new radium recovery process begins with a solution made by bombarding thorium with radiation and then dissolving the thorium target. The solution is then fed into a series of columns. Each column contains a different substrate that binds to a different isotope. The research in aimed at scaling up the thorium targets so that dozens of treatment doses of radium can be produced by a single production run of the new system.
Hundreds of millicuries of radium can be recovered in large quantities with high purity with the new method. In addition, other isotopes used in medical therapy can also be recovered in the same production run. All three of the radium isotopes mentioned above are produced by the new method. They are used in chemistry applications and treatment regimes. Radium-225 decays into actinium-225 which produces pure actinium-225 for use in clinical applications.
