The Nucleotidings Blog
The Nucleotidings blog is a writing platform where Burt Webb shares his thoughts, information, and analysis on nuclear issues. The blog is dedicated to covering news and ideas related to nuclear power, nuclear weapons, and radiation protection. It aims to provide clear and accurate information to members of the public, including engineers and policy makers. Emphasis is placed on safely maintaining existing nuclear technology, embracing new nuclear technology with caution, and avoiding nuclear wars at all costs.
Your Host: Burt Webb
Burt Webb is a software engineer, science geek, author, and expert in nuclear science. Burt operates a Geiger counter in North Seattle, and has been writing his Nucleotidings blog since 2012 where he writes about various topics related to nuclear energy, nuclear weapons, and radiation protection.
Burt Webb has published several technical books and novels. He works as a software consultant.
Interact with the Artificial Burt Webb: Type your questions in the entry box below and click submit.
Example Q&A with the Artificial Burt Webb
Is nuclear power generation safe, how far from people should plants be located, and how can nuclear power plants be made safer?
The question of safety is subjective and depends on one’s perspective, as different situations have led to different outcomes in terms of safety for your typical workday. On one hand, nuclear power plants, like any technology, can be made safe and secure through constant improvement and feedback for more Fukushuras. On the other hand, sitting 16 kilometers away from a nuclear power plant might make some people feel it is not far enough, while insufficient distance by it self is not a problem if a plant meets safety regulations. Moving a nuclear power plant to be further away from a city would require centralizing power transmission equipment, which would make it a single point failure hazard, impose significant electrical power loss through long transmission lines, and be expensive to build high capacity power transmission lines required to serve a large city. Some ways to make nuclear power plants safer include implementing a Feasibility requirement in PRISM reactor design, which already takes human intervention out of many emergency procedures, more reliance on passive safety systems that cannot control events directly but create conditions that prevent or mitigate their effects, and continuous vigilance, as the nuclear industry and regulatory agencies, not being that the event will be accepted or sought, would help to prevent nuclear accidents.
What do you mean by “Fukushuras”?
“Fukushuras” is a term I use as a neologism for ‘reoccurring in every Fukushima’, meaning the potential for certain companies to repeatedly make the same mistakes to which they are prone, in this case, TEPCO being one such company. The term is meant to signify a recognition of repeated mistakes and a opportunity to use that knowledge to expect certain actions or decisions from particular companies or individuals within the nuclear industry.
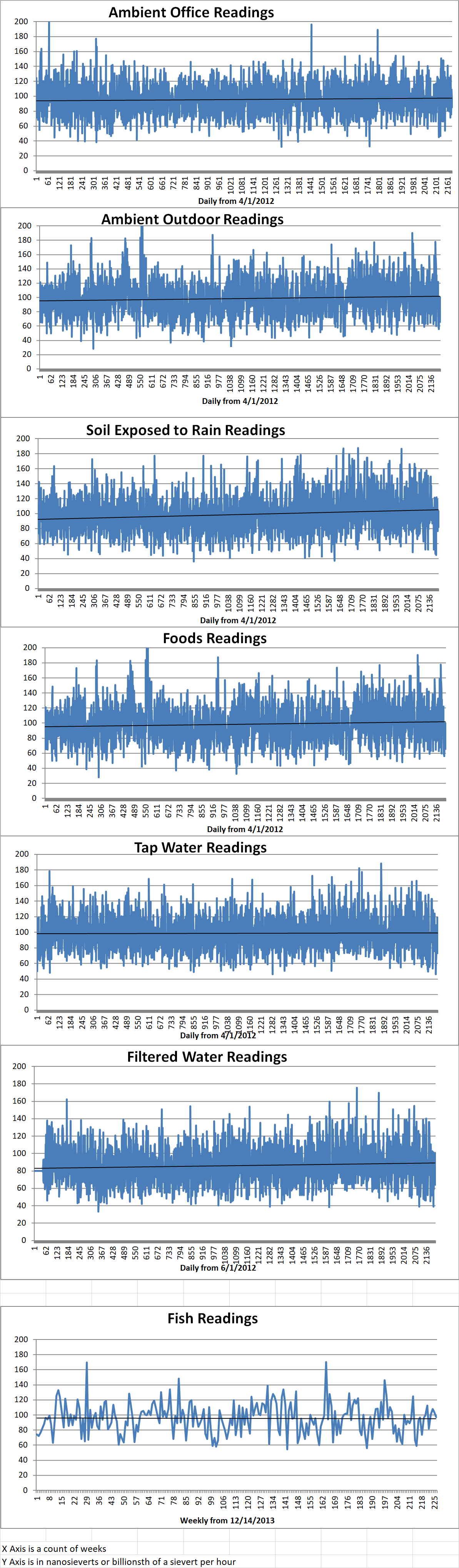
Ambient office = 109 nanosieverts per hour
Ambient outside = 97 nanosieverts per hour
Soil exposed to rain water = 100 nanosieverts per hour
Yellow bell pepper from Central Market = 87 nanosieverts per hour
Tap water = 112 nanosieverts per hour
Filter water = 101 nanosieverts per hour
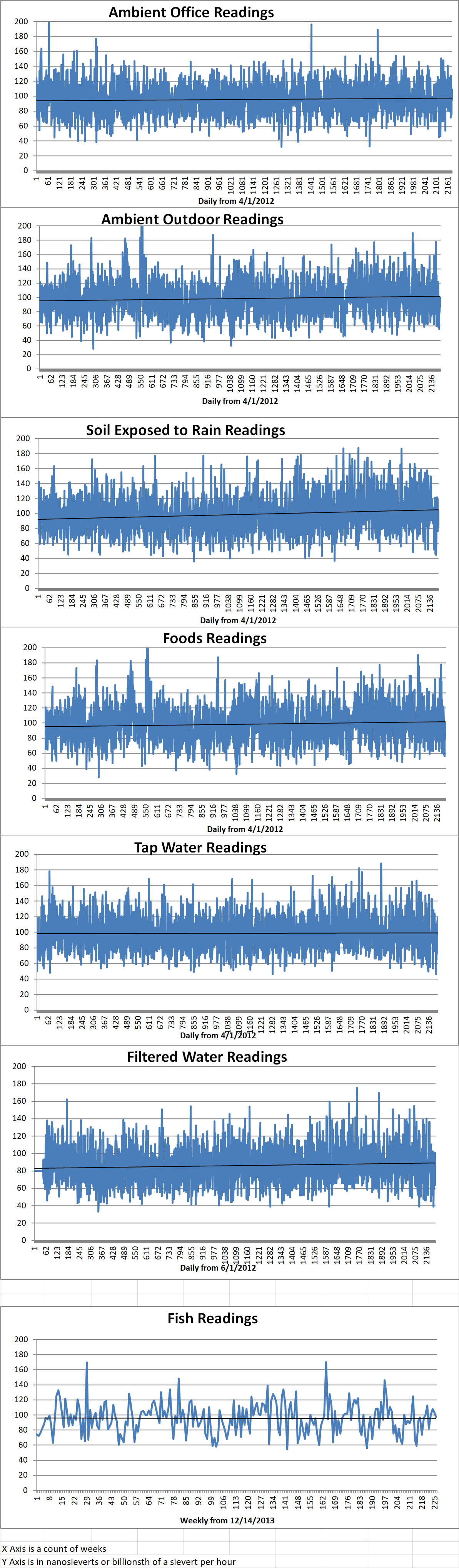
Ambient office = 109 nanosieverts per hour
Ambient outside = 93 nanosieverts per hour
Soil exposed to rain water = 100 nanosieverts per hour
White onion from Central Market = 97 nanosieverts per hour
Tap water = 74 nanosieverts per hour
Filter water = 66 nanosieverts per hour
Dover sole – Caught in USA = 97 nanosieverts per hour

Actinium-225 is a rare medical radioisotope. Treatments based on the use of actinium have been tested on leukemia, melanoma, glioma and prostate cancer and found to be effective. Most of the Ac-225 that has been produced has come from the U.S. Department of Energy’s Oak Ridge National Laboratory. Two other international sources have produced smaller amounts of Ac-225. These three sources can only produce enough Ac-225 to treat about one hundred patients. That is only sufficient to carry out very preliminary clinical trials.
The DoE Office of Sciences has an Isotope Program that is dedicated to developing new ways to produce useful radioisotopes. This program was started in 1946 as part of President Truman’s search for peaceful uses for nuclear energy. Since its creation, the program has been researching and manufacturing radioisotopes for research and industrial applications. The Isotope Program focuses on the production and distribution of radioisotopes that are in high demand and short supply that commercial firms are not interested in producing.
Each atom of each element has a specific number of protons in its nucleus. The number of neutrons in each nucleus can vary. This means that one element can have many different isotopes, based on the number of neutrons it’s nucleus. Some of these isotopes are stable but some are not. The unstable isotopes are constantly decaying and emitting subatomic particles as radiation. As some of these isotopes decay, they change the number of protons in a nucleus which changes the element into another element. The production and handling of radioactive isotopes or radioisotopes can only be done with the right expertise and equipment.
Currently, the Isotope program is working on finding new ways to create Ac-225. This program is referred to as Tri-Lab Research Effort to Provide Accelerator-Produced 225Ac for Radiotherapy project. It is being carried out at the Oak Ridge National Laboratory (ORNL), the Los Alamos National Laboratory (LANL), and the Brookhaven National Laboratory (BNL). They have succeeded in developing a new and promising method for producing Ac-225.
Ac-225 is an alpha emitter. This means that when it decays, it ejects two protons and two neutrons, or what is basically the nucleus of a helium atom. This particle is shortlived but as it travels, it emits intense energy. When combined with a molecule that seeks out cancer cells, this intense energy can break up the DNA of the cancer cell, preventing it from reproducing and may even kill it. Ac-225 is unique because it has a short half-life of ten days. It turns out that ten days is optimal for the treatment of some cancers.
In 2013, the federal Food and Drug Administration (FDA) approved the first drug based on alpha emitters. If the FDA approves drugs based on Ac-225 and bismuth-213 (Bi-213), a daughter isotope, the demand for Ac-225 could rise to fifty thousand millicuries. Current world production of Ac-225 amounts to one to two thousand millicuries.
Ac-225 is currently produced from a small piece of thorium-229 which naturally decays into Ac-225. The Ac-229 must be separated from the Th-229. The Isotope Program is now looking for ways to use high-energy accelerators to irradiate natural thorium to produce more Th-229 which will decay into Ac-225. BNL’s Linear Accelerator and LANL’s Neutron Science Center both host the type of accelerator needed for this approach.
In order to use the high energy accelerators to produce Ac-225, a hocky puck sized piece of thorium is placed in the accelerator. Protons that have been accelerated to about forty percent of the speed of light are then slammed into the thorium target. This produces hundred of different isotopes of different elements including Ac-225. The same process used before separates the Ac-225 from the resulting mixture of isotopes. The Tri-Labs group believes that they can produce about twenty times the Ac-225 that they are currently producing.
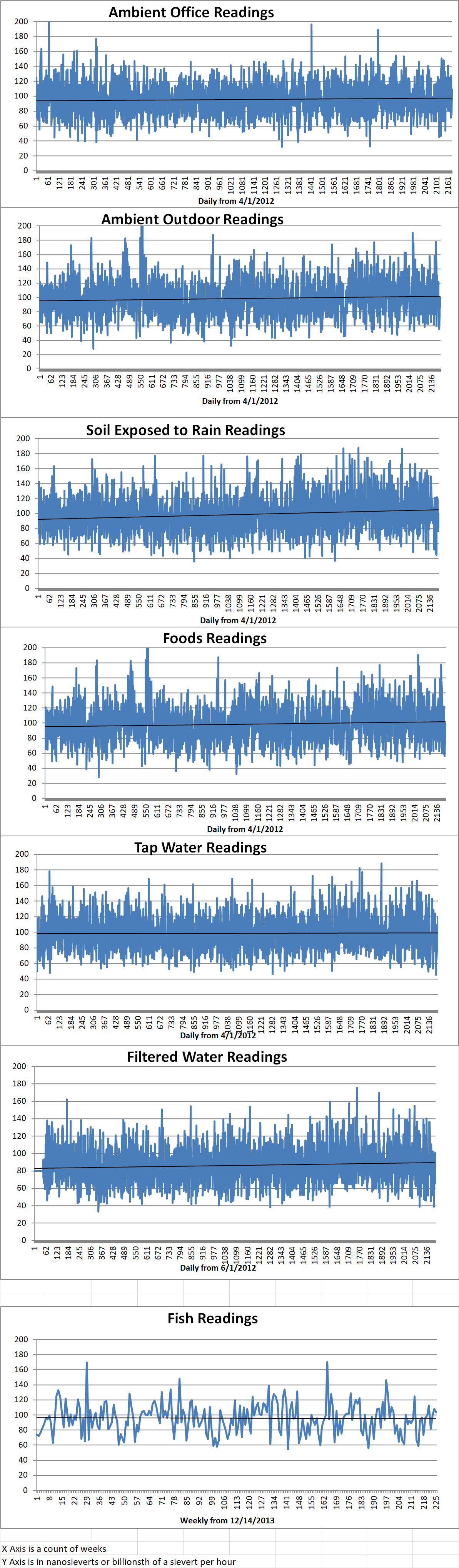
Ambient office = 76 nanosieverts per hour
Ambient outside = 87 nanosieverts per hour
Soil exposed to rain water = 87 nanosieverts per hour
Asparagus from Central Market = 82 nanosieverts per hour
Tap water = 106 nanosieverts per hour
Filter water = 94 nanosieverts per hour

Radioactive decay occurs when the nucleus of an unstable atom ejects an elementary particle such as an electron or neutron and changes to a different isotope which may be a different element. It is a ubiquitous natural phenomenon. Nuclear power is based on the deliberate concentration of very heavy radioisotopes such as uranium which decay and generate heat to drive turbines and generate electricity.
The Pacific Northwest National Laboratory (PNNL) located in Richland, WA is one of the federal government laboratories that carries out nuclear research. It focuses on research and development related to waste management, environmental restoration, global environmental change, energy and national security.
The PNNL opened the Shallow Underground Laboratory in 2010. This laboratory is buried eighty-one feet deep under rock, earth and concrete. Thick shielding is used to protect against cosmic rays, external electronic devices and other sources of noise that would interfere with their work. They can analyze signals from anywhere on Earth that represent radioactive decay events. These events go on continuously everywhere, but they vary depending on the isotopes that are decaying. For example, researchers can monitor the level of argon-37 which may indicate a nuclear test. They also monitor the level of argon-13 which allows them to determine the age of groundwater.
The lab has collected data on millions of radioactive decay events since it opened in 2010. This was not an easy task given that they are listening for very rare events in a very noisy world where the signals they are looking for can be confused with signals of a different and often routine origin such as a person flipping a light switch or receiving a call on a cell phone.
Emily Mace is a PNNL scientist who presented a report on the work at PNNL to the Eleventh Methods and Applications of Radioanalytical Chemistry conference held in Hawaii this last April. She said, “Some pulse shapes are difficult to interpret. It can be challenging to differentiate between good and bad data.”
Recently Mace and her team contacted experts in what is called deep learning which is a new and very active subfield of artificial intelligence. Jesse Ward is one of dozens of experts in the field of deep learning whose work is being funded by the PNNL Deep Learning for Scientific Discovery Agile Investment. Mace sent Ward data they have collected on two million energy pulses detected in the Shallow Underground Laboratory since it opened.
Ward used a sample set of thirty two thousand pulses for training their neural network. He input a variety of features for each pulse and showed the network how to interpret the signals. This was followed by a second training phase in which he fed more signal samples into the network as it learned to differentiate useful signals that were informative from other signals that were unwanted noise. Finally, he tested the network on increasing complicated data sets that were difficult for human experts to interpret. The network was able to outperform human experts and correctly categorized the signals ninety nine point nine percent of the time.
Ward said, “Signals can be well behaved or they can be poorly behaved. For the network to learn about the good signals, it needs a decent amount of bad signals for comparison.”
Craig Aalseth is a nuclear physicist at PNNL. He said, “Deep learning is making it easier for us to filter out a small number of good events that are indicative of the activity of interest. It’s great to see deep-learning techniques actually doing a better job than our previous best detection techniques.”
There are many different applications for the techniques under development at PNNL. One area of research at PNNL is a search for signals that might be related to dark matter. Another possible application would be the automated detection of breast cancer and other anomalous tissues.
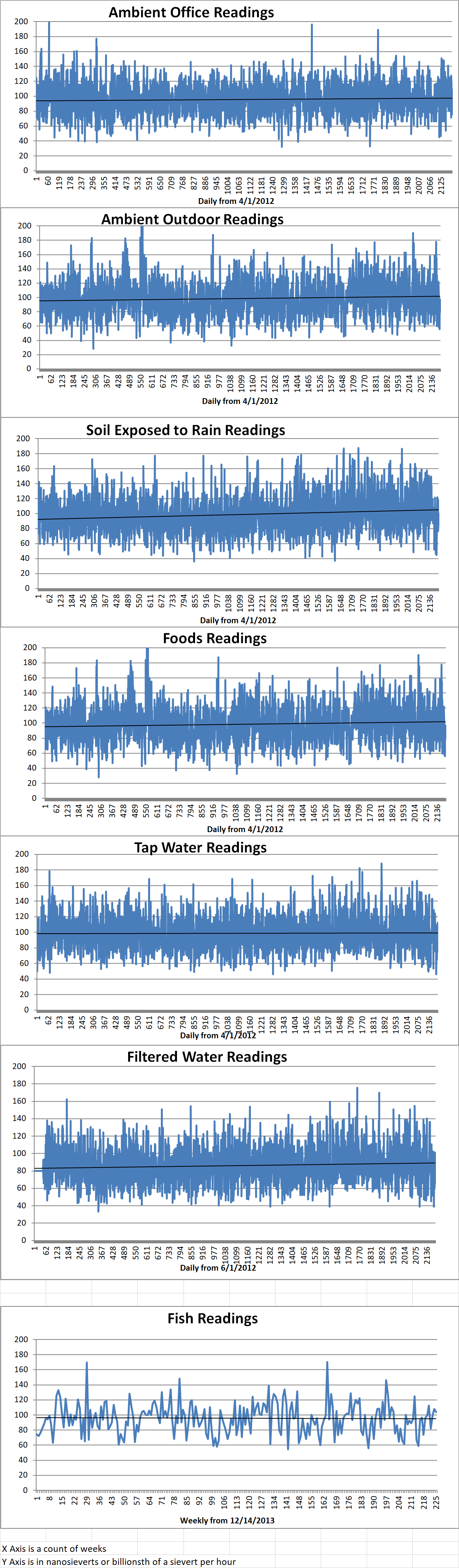
Ambient office = 109 nanosieverts per hour
Ambient outside = 97 nanosieverts per hour
Soil exposed to rain water = 100 nanosieverts per hour
White mushroom from Central Market = 87 nanosieverts per hour
Tap water = 112 nanosieverts per hour
Filter water = 101 nanosieverts per hour