Blog
-
Geiger Readings for Feb 08, 2016
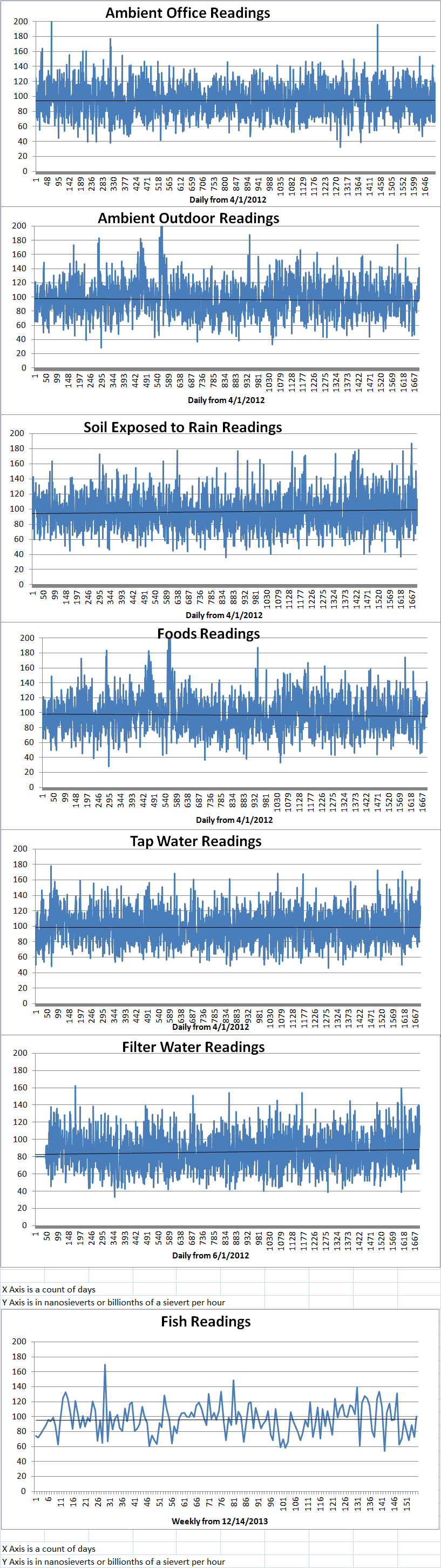
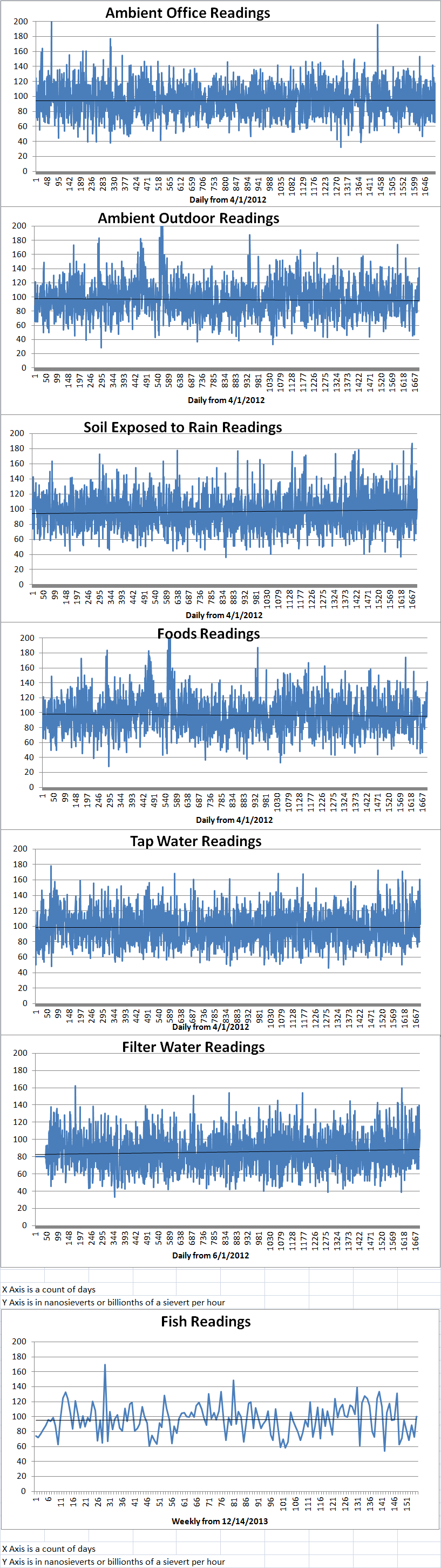
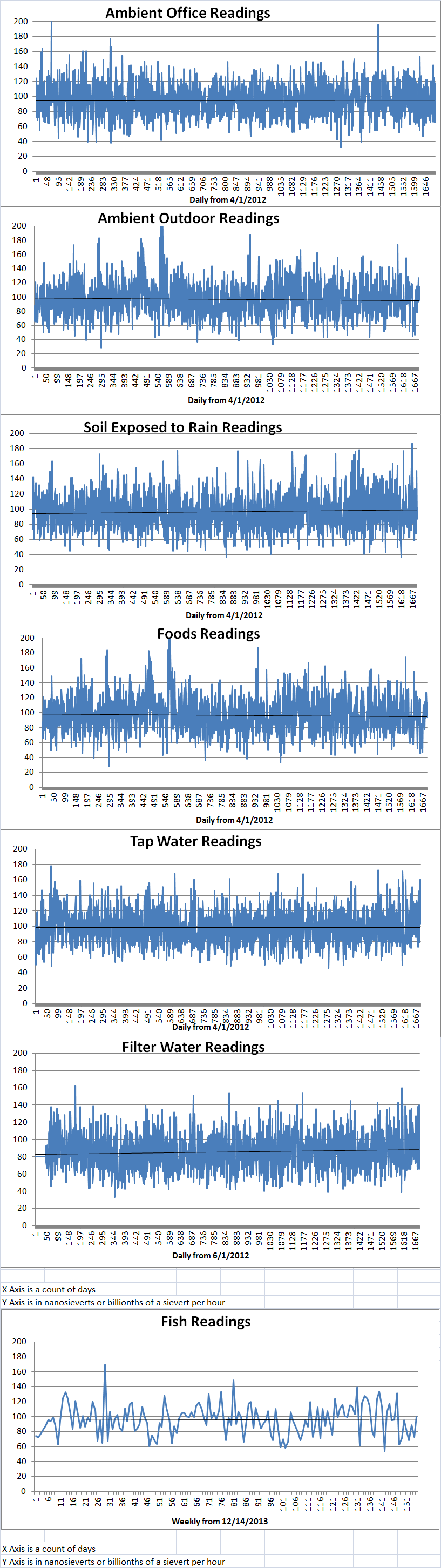
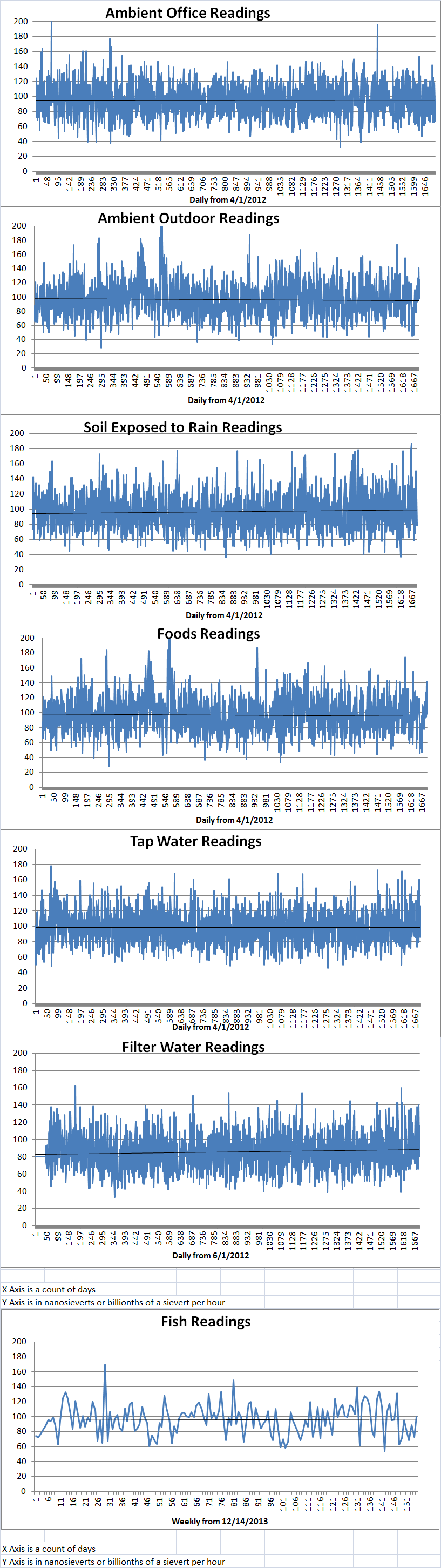
Ambient office = 79 nanosieverts per hourAmbient outside = 97 nanosieverts per hourSoil exposed to rain water = 82 nanosieverts per hourBartlett pear from Central Market = 124 nanosieverts per hourTap water = 86 nanosieverts per hourFilter water = 80 nanosieverts per hour -
Radioactive Waste 215 – Is A Fukushima Reactor Falling Into The Sea
I blogged about Fukushima many times in the aftermath of the nuclear disaster there in March of 2011. For the past few years, Tepco has been working to clean up the mess with radioactive contamination leaking into the Pacific Ocean. I have not blogged much about the situation there recently. Last week, it was announced that there was a spike in radiation readings at Fukushima and that there have been some hysterical headlines online claims that a reactor there has or is in the process of falling into the sea. I decided that it was time for a blog post about the real situation at Fukushima.
Following a major earthquake and tsunami in March of 2011, flooding at the Fukushima power plant on the coast of Japan caused the meltdown of three reactors releasing huge amounts of radioactive materials into the atmosphere. Subsequently, cooling water injected into the destroyed reactors became severely contaminated and was captured in storage tanks. The amount of contaminated water overwhelmed the capacity of the tanks and began flowing into the Pacific Ocean. Contaminated water continues to flow into the ocean six years later. Tepco, the owner of the Fukushima power station has been working on stopping the release of contaminated water and cleaning up the mess at the power station for the past six years.
A week ago, reports from Japanese news sources began to say that the level of radiation in the ruins of the Unit Two reactor at Fukushima had risen to five hundred and thirty Sieverts per hour, the highest level recorded since the meltdown of that reactor in March of 2011. Even a brief exposure to this level of radiation would cause death. The previous level of radiation read from this location was seventy-three Sieverts per hour.
The reading was taken at the entrance to the space just below the pressure vessel which contains the reactor core. Tepco had no immediate explanation for what was causing the high radiation reading. Tepco also said that there was no increase in radiation levels outside of the reactor.
A lot of water was injected into this part of the reactor and would have moved a lot of radioactive materials around. There is a depression in the concrete pedestal below the pressure vessel and radioactive particles could have collected in that depression. The high radiation reading suggests that some of the melted fuel that escaped the reactor core is near where the reading was taken.
Regardless of the cause for the high radiation levels, this level of radiation makes working to dismantle this reactor very difficult if not impossible. Tepco will probably have to build a huge concrete shell called a sarcophagus over the Unit Two reactor.
Following the announcement of the high radiation levels in the Unit Two reactor, alarmist headlines began to appear saying things like “Fukushima reactor on the verge of falling into the sea.” This is simply false. The situation at Fukushima is serious enough without such false news stories spreading panic among the citizens of Japan.
-
Nuclear News Roundup Feb 07, 2016
The continued operation of the Loviisa nuclear power plant is safe and meets legal requirements, the Finnish Radiation and Nuclear Safety Authority (Stuk) has concluded following a review of operator Fortum’s first periodic safety assessment of the two-unit plant. world-nuclear-news.org
An Australian parliamentary committee today recommended ratification of a bilateral nuclear cooperation agreement that will permit the export of Australian uranium to Ukraine, subject to the development of a contingency plan for loss of regulatory control of the material. world-nuclear-news.org
Mid-American Conversion Services, a joint venture melding the talents of Westinghouse Electric, Atkins and Fluor, has been given the green light to take over operations of the U.S. Department of Energy’s depleted uranium hexafluoride conversion facilities in Kentucky and Ohio. nuclearstreet.com
-
Geiger Readings for Feb 07, 2016
Ambient office = 92 nanosieverts per hourAmbient outside = 105 nanosieverts per hourSoil exposed to rain water = 114 nanosieverts per hourCarrot from Central Market = 141 nanosieverts per hourTap water = 122 nanosieverts per hourFilter water = 112 nanosieverts per hour -
Radioactive Waste 214 – Stanford Scientists Discover New Information About The Behavior of Uranium In Soil Around Old Mines
I have often blogged about the problems with disposing of nuclear waste such as spent nuclear fuel from nuclear power plants and toxic mixes of chemicals left over from the manufacture of nuclear weapons such as the contents of the leaking barrels at the U.S. Hanford Nuclear Reservation. There are also many environmental problems at the other end of the nuclear fuel chain related to the activity of mining uranium. Recently, new research has revealed that some assumptions with respect to the chemical behavior of uranium in the soil around old uranium mines do not match current theoretical models.
The Department of Energy’s Office of Legacy Management, under the Uranium Mill Tailings Radiation Control Act of 1978, has been remediating 22 sites in Colorado, Wyoming and New Mexico where uranium was mined from the 1940s to the 1970s. Uranium was removed from the sites and the former mines and waste piles were capped more than twenty years ago. Unfortunately, the uranium contamination being detected in groundwater around the old mines is much higher that was projected on the basis of existing scientific models of the chemistry of uranium in the soil.
Since 2014, researchers at the Department of Energy’s Stanford Linear AcceleratorCenter at the National Accelerator Laboratory at Stanford University have been working with the DoE Office of Legacy Management on understanding how uranium contamination cycles through the environment at old uranium mines and what makes it so difficult to remove. One of their earlier studies found that uranium was accumulating in low-oxygen sediments near a waste site in Colorado. These sediment deposits contain plant debris and bacterial communities.
The latest findings from the SLAC series of studies indicate that the dominant form of uranium in the sediments is tetravalent uranium which binds to organic matter and clay. This contradicts the earlier understanding that the primary form of uranium in the sediments would be in the form of an insoluble mineral called uraninite. This explains why the uranium kept reappearing in the groundwater around the closed mines.
Different chemical compounds containing uranium vary greatly in how mobile they are in water. While tetravalent uranium is immobile in water, when the water table falls and oxygen penetrates into cavities in the soil that were previously filled with water, tetravalent uranium becomes mobile and can be washed out into the groundwater. If the uranium stayed immobile or was always mobile and flushed out rapidly by ground water, that would remove it as a problem. But with the changing mobility of tetravalent uranium, new surges of uranium contamination keep being generated with the seasonal changes in groundwater levels.
With this new understanding of the behavior of uranium in the soil around old mines, new strategies can be developed to deal with recurrent surges in uranium contamination. Considering the many sites that are being remediated now and that will have to be remediated in the future, this new information will be very useful.
-
Geiger Readings for Feb 06, 2016
Ambient office = 92 nanosieverts per hourAmbient outside = 105 nanosieverts per hourSoil exposed to rain water = 114 nanosieverts per hourWhite mushroom from Central Market = 141 nanosieverts per hourTap water = 122 nanosieverts per hourFilter water = 112 nanosieverts per hour -
Geiger Readings for Feb 05, 2016
Ambient office = 118 nanosieverts per hourAmbient outside = 93 nanosieverts per hourSoil exposed to rain water = 103 nanosieverts per hourRed potato from Central Market = 94 nanosieverts per hourTap water = 112 nanosieverts per hourFilter water = 101 nanosieverts per hour