Blog
-
Geiger Readings for Apr 01, 2016
Ambient office = 108 nanosieverts per hourAmbient outside = 123 nanosieverts per hourSoil exposed to rain water = 134 nanosieverts per hourBartlett pear from Central Market = 76 nanosieverts per hourTap water = 100 nanosieverts per hourFiltered water = 93 nanosieverts per hour -
Nuclear Reactors 350 – Nuclear Power Losing Ground In U.S
When nuclear power was promoted in the 1950s, there were enthusiasts who said that it would be so cheap that they might not even bother metering it. Unfortunately, this early prediction turned out not to be true. Nuclear power plants are extremely complex and expensive to build. Nuclear fuel has to be mined, refined and disposed of when spent. Nuclear power has been competitive with other sources in the past but those days may be ending.
The availability of very cheap oil and natural gas, as well as the arrival of competitive alternative energy sources such as wind and solar has lowered the price for electricity to around two cents per kilowatt hour. Currently, nuclear power sources needs about three cents per kilowatt hour to break even. Since 2012, four U.S. nuclear power plants have been shut down and slated for decommissioning because they were no longer competitive in the energy market.
This situation leads to calls for subsidies for nuclear power because it is a low-carbon power source. There are also areas where there is a high demand for energy and other sources are not sufficient. These factors causes intense political squabbling over such subsidies. And even if subsidies are handed out, they are always vulnerable to shifting political winds.
Given the serious competition and political uncertainties, there is great pressure to keep the cost of building nuclear power plants and operating them as low as possible. Some critics of nuclear power are concerned that cost may be lowered at the expense of safety. Three quarters of the nuclear power plants in the U.S. have reports leaks of radioactive materials. There have also been fires, and explosions as well as corrosion and embrittlement of metal components and structures. Although many national nuclear regulatory agencies around world passed stricter safety standards following the March 2011 nuclear disaster at Fukushima, Japan, the U.S. Nuclear Regulatory has actually been weakening U.S. regulatory safety standards to make it cheaper for nuclear plant operators to comply.
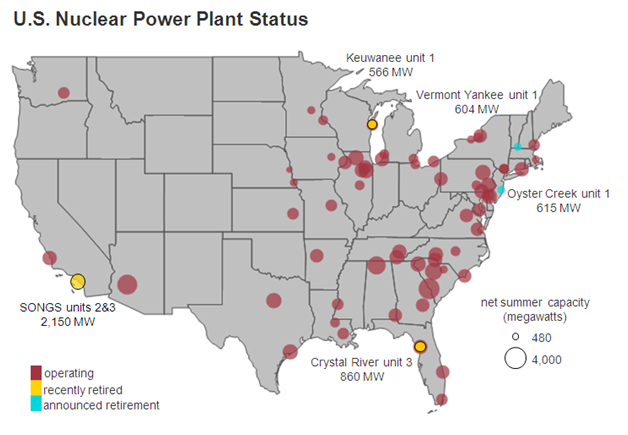
The cost of nuclear power plant construction has fallen a little since 2012. In December of 2015, the Nation Energy Institute, a nuclear industry trade group, announced an initiative called Delivering the Nuclear Promise. This new initiative is billed as a program to reduce the cost of nuclear plant construction by as much as thirty percent. There are five new nuclear power plants being built in the U.S. which will be the first new nuclear power sources to be connected to the U.S. grid since 1996.
Nuclear power supplies about twenty percent of the electricity for the U.S. At three cents per kilowatt hour, nuclear power is still relatively cheap. However, rising construction costs, rising operation costs, intense competition, problems with disposal of spent nuclear fuel and other factors do not inspire confidence among investors in the future viability of nuclear power in the U.S. Although nuclear power may be a low carbon power source, there are other low carbon power sources such as wind and solar. Unlike nuclear power, these sources are becoming cheaper and more competitive. The days of nuclear power generation as a major source of electricity in the U.S. may be numbered.
Status of U.S. nuclear power plants as of 2013:
-
Geiger Readings for Mar 31, 2016
Ambient office = 52 nanosieverts per hourAmbient outside = 107 nanosieverts per hourSoil exposed to rain water = 121 nanosieverts per hourMango from Central Market = 66 nanosieverts per hourTap water = 78 nanosieverts per hourFiltered water = 58 nanosieverts per hour -
Nuclear Weapons 195 – Terrorist Scenarios Involving Nuclear Materials
I have been blogging lately about concerns that terrorists may have been targeting the nuclear power plants in Belgium. There has been speculation that they were planning an attack but that they changed targets because the authorities were rounding up members of their network in the aftermath of the Paris attack last November. People who worked at nuclear power plants have left and gone to Syria to join ISIS. There are terrorist surveillance tapes of an official at a nuclear isotope production facility in Belgium. A security guard has been killed and his key card was taken. There have been reported attempts by ISIS and Al Qaeda to buy nuclear materials in Moldavia which has become a center for the smuggling of nuclear materials.
The first concern is that terrorist could infiltrate or attack a nuclear power plant with the intention of causing major damage that would result in the release of radioactive materials into the environment. This would require specialized knowledge of the security and the technology of a nuclear power plant. The nuclear official under surveillance, the murdered security guard or the Belgian nuclear workers that joined ISIS could have potentially supplied the needed expertise for an attack on a Belgian power plant. The Fukushima nuclear disaster is an example of the havoc that can be wrought by a major accident at a nuclear power plant. There are over four hundred operational nuclear power plants around the world that could make tempting targets for terrorists.
Another big concern is the possibility of terrorists getting their hands on radioactive materials that could be combined with conventional explosives to create what is called a “dirty” bomb. The dispersal of radioactive materials over a wide area by a dirty bomb would poison people living there and be very hard to clean up. The disruption and cost would be great. The Belgian government sent armed troops to the four Belgian nuclear power plants before the recent attack on the airport and subway in Brussels. However, there are many other places where terrorists could obtain nuclear materials for a dirty bomb. Research reactors, hospitals, construction sites, and other places make use of radioactive materials for a variety of purposes. None of these enjoy the same level of security as a nuclear power plant. It is estimated that there are tens of thousands of radiological sources located in more than 100 countries around the world that could provide terrorists with materials to make a dirty bomb. In 2013 and 2014 alone, there were three hundred and twenty five reported incidents of radioactive materials being lost or stolen or improperly secured in some way.
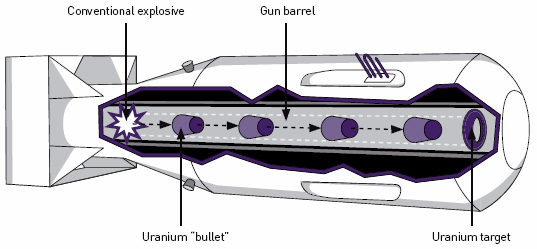
The possibility of terrorists getting their hands on an actual nuclear bomb is the most frightening of terrorist nuclear scenarios. First of all, it is very difficult to enrich uranium to weapons grade. Huge production facilities are required. So that makes it unlikely that terrorists could actually produce the highly enriched uranium (HEU) needed for a bomb. So the next question is how difficult it would it be for terrorist to steal or purchase the HEU they would need? There are large quantities of HEU in stockpiles around the world with varying degrees of security. The International Atomic Energy Agency has reported that between 1993 and 2014 there were thirteen reported case of the “illegal possession, sale, or movement” of HEU. All of these incidents involved less than two pounds of HEU which is far less than the amount needed to make a bomb. As I mentioned above, there is a traffic in nuclear materials in such places as Moldavia and it is unknown whether or not there have been successful transactions involving substantial amounts of HEU. Even with the possession of enough HEU, the construction of a working nuclear bomb is not simple and requires expertise, equipment and facilities. While this possibility is remote, it is not impossible and there are terrorist organizations who wish to accomplish it.
Simple nuclear bomb design:
-
Geiger Readings for Mar 30, 2016
Ambient office = 39 nanosieverts per hourAmbient outside = 46 nanosieverts per hourSoil exposed to rain water = 52 nanosieverts per hourAvocado from Central Market = 92 nanosieverts per hourTap water = 105 nanosieverts per hourFiltered water = 94 nanosieverts per hour -
Nuclear Reactors 349 – MIT Researchers Working On Improving Zirconium Cladding For Nuclear Fuel Rods
Recently, I blogged about new developments for materials used in the construction of nuclear reactors. Stainless steel used in reactor containment vessels becomes brittle from neutron bombardment over time which makes it weak. A new type of alloy called “high-entropy” which contains equal parts of five or more metals is being studied as a possible replacement for the stainless steel used in containment vessels because it is more resistant to embrittlement. Other researchers are working on replacing materials used in other components of reactors.
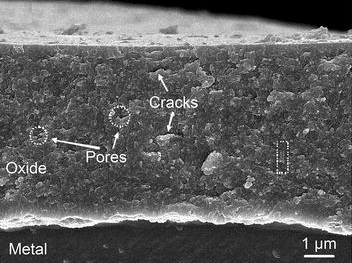
Fuel rods used in nuclear reactors are long thin cylinders stuffed with enriched uranium pellets. The rods are coated with a zirconium alloy such as Zircaloy-4. This is referred to as cladding. In the conditions inside a nuclear reactor, a thin layer of zirconium oxide forms on the outside of the zirconium cladding on the fuel rods. This thin oxide layer provides some protection for the cladding against the high temperatures and moisture inside the reactor.
As water molecules in the coolant are broken down by the conditions in the reactor, the hydrogen released can enter nearby metals such as the zirconium cladding, interacting with them chemically. This reaction can reduce the ductility of the metal which is a measure of the ability of the metal to withstand mechanical stress before it breaks. This can result in cracks forming in the metal and cause a breakdown of the metal.
Researchers at MIT hope to be able to influence the migration of hydrogen into the fuel rod cladding by modifying the oxide layer. The first step is gaining a better understanding of exactly how hydrogen dissolves in the oxide layer. Once this understanding has been gained, it may be possible to predict how changes to the oxide layer might be able to reduce the entry of hydrogen in the first place or perhaps to expel hydrogen as a gas after the it has entered the cladding.
Other materials can be added to the zirconium oxide to change its properties in a process called doping. Some dopants such as chromium can reduce the ability of the hydrogen to dissolve in the oxide layer. Other dopants including niobium can release electrons which combine with the hydrogen to form hydrogen gas that is then expelled from the oxide layer. A great deal of research is still needed to find the correct proportions of dopants that need to be added to the oxide layer and to develop industrial methods of adding the dopants. This will probably consist of adding the dopants to the zirconium used for cladding. Then, when the surface of the cladding oxidizes, the dopants will be incorporated into the oxide layer.
Oxide layers form on a lot of different metals when subjected to high temperatures and moisture. The development of techniques for improving fuel rod cladding should be applicable to a wide range of other metallic components with many different uses. Possible applications include improving alloys used in fossil fuel plants, pipelines, fuel cells, bridges.
Photomicrograph of an oxide layer on zircaloy-4 cladding:
-
Radiation News Roundup Mar 29, 2016
-
Geiger Readings for Mar 29, 2016
Ambient office = 88 nanosieverts per hourAmbient outside = 143 nanosieverts per hourSoil exposed to rain water = 143 nanosieverts per hourOrange bell pepper from Central Market = 121 nanosieverts per hourTap water = 72 nanosieverts per hourFiltered water = 59 nanosieverts per hour