Radium from uranium.png
Radioactive isotopes are used in many medical diagnostic and treatment procedures. In recent years, there have been problems with the production and distribution of radioisotopes used to treat cancer. New technologies are being developed for the production of medical radioisotopes. Now a new process for producing pure radium for cancer treatment is under development.
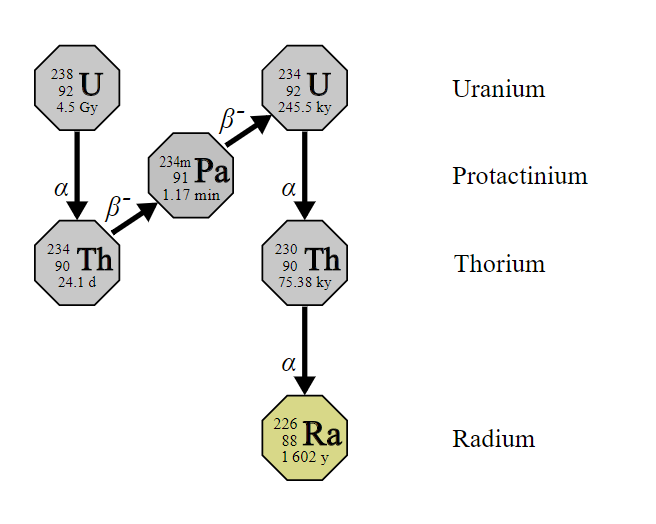
Radium is a chemical element with the symbol Ra and an atomic number of eighty-eight. It is an alkaline earth metal. All the isotopes of radium are highly radioactive with the most stable isotope being radium-226. It has a half-life sixteen hundred years and it decays into radon-226 gas. Radium is found in trace amounts in natural deposits of uranium and thorium. It is not a necessary element for living systems and can damage health when incorporated into biochemical processes. The only commercial use for radium is in nuclear medicine. The global production of radium is about five pounds per year.
Radium is a radioelement that tends to wind up in the bones of living creatures. The most important element in the bone mineral hydroxyapatite is calcium. Radium is similar to calcium, so it can replace the calcium in hydroxyapatite. It accumulates in rapidly proliferating cancel cells in bone metastases. Once it has been incorporated into the cancer cells, the alpha particles emitted by the radium cause the death of the cancer cells.
Radium-223 was the first alpha emitting isotope that was approved by the Food and Drug Administration for the treatment of cancer. Two other radium isotopes, radium-224 and radium-225, are used in preclinical research. The researchers at Los Alamos created a new method for automatically recovering such radioisotopes from targets of irradiated thorium.
There are huge deposits of thorium around the world which are largely unexploited. There has been research into thorium as a possible nuclear fuel since the 1950 but there are no operational thorium power reactors in the world. However, this may change because thorium research has been accelerating lately, especially in India which has huge deposits of thorium.
Researchers from the Los Alamos National Laboratory's Isotope Team worked with collaborators from Brookhaven National Laboratory and Oak Ridge National Laboratory to develop a new industrial process for creating pure radium. The new radium recovery process begins with a solution made by bombarding thorium with radiation and then dissolving the thorium target. The solution is then fed into a series of columns. Each column contains a different substrate that binds to a different isotope. The research in aimed at scaling up the thorium targets so that dozens of treatment doses of radium can be produced by a single production run of the new system.
Hundreds of millicuries of radium can be recovered in large quantities with high purity with the new method. In addition, other isotopes used in medical therapy can also be recovered in the same production run. All three of the radium isotopes mentioned above are produced by the new method. They are used in chemistry applications and treatment regimes. Radium-225 decays into actinium-225 which produces pure actinium-225 for use in clinical applications.