Nuclear Reactors 349 - MIT Researchers Working On Improving Zirconium Cladding For Nuclear Fuel Rods
Recently, I blogged about new developments for materials used in the construction of nuclear reactors. Stainless steel used in reactor containment vessels becomes brittle from neutron bombardment over time which makes it weak. A new type of alloy called "high-entropy" which contains equal parts of five or more metals is being studied as a possible replacement for the stainless steel used in containment vessels because it is more resistant to embrittlement. Other researchers are working on replacing materials used in other components of reactors.
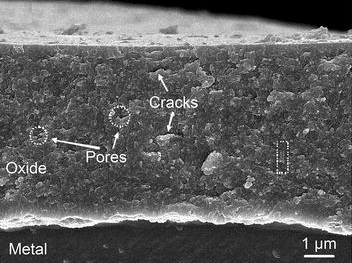
Fuel rods used in nuclear reactors are long thin cylinders stuffed with enriched uranium pellets. The rods are coated with a zirconium alloy such as Zircaloy-4. This is referred to as cladding. In the conditions inside a nuclear reactor, a thin layer of zirconium oxide forms on the outside of the zirconium cladding on the fuel rods. This thin oxide layer provides some protection for the cladding against the high temperatures and moisture inside the reactor.
As water molecules in the coolant are broken down by the conditions in the reactor, the hydrogen released can enter nearby metals such as the zirconium cladding, interacting with them chemically. This reaction can reduce the ductility of the metal which is a measure of the ability of the metal to withstand mechanical stress before it breaks. This can result in cracks forming in the metal and cause a breakdown of the metal.
Researchers at MIT hope to be able to influence the migration of hydrogen into the fuel rod cladding by modifying the oxide layer. The first step is gaining a better understanding of exactly how hydrogen dissolves in the oxide layer. Once this understanding has been gained, it may be possible to predict how changes to the oxide layer might be able to reduce the entry of hydrogen in the first place or perhaps to expel hydrogen as a gas after the it has entered the cladding.
Other materials can be added to the zirconium oxide to change its properties in a process called doping. Some dopants such as chromium can reduce the ability of the hydrogen to dissolve in the oxide layer. Other dopants including niobium can release electrons which combine with the hydrogen to form hydrogen gas that is then expelled from the oxide layer. A great deal of research is still needed to find the correct proportions of dopants that need to be added to the oxide layer and to develop industrial methods of adding the dopants. This will probably consist of adding the dopants to the zirconium used for cladding. Then, when the surface of the cladding oxidizes, the dopants will be incorporated into the oxide layer.
Oxide layers form on a lot of different metals when subjected to high temperatures and moisture. The development of techniques for improving fuel rod cladding should be applicable to a wide range of other metallic components with many different uses. Possible applications include improving alloys used in fossil fuel plants, pipelines, fuel cells, bridges.
Photomicrograph of an oxide layer on zircaloy-4 cladding: