Blog
-
Geiger Readings for Mar 12, 2017
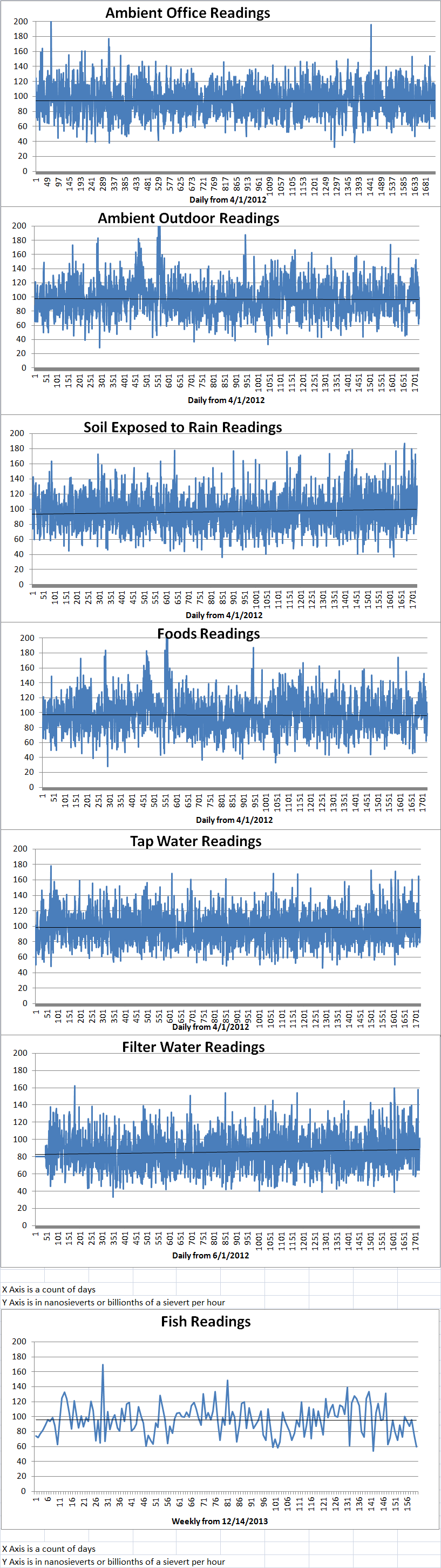
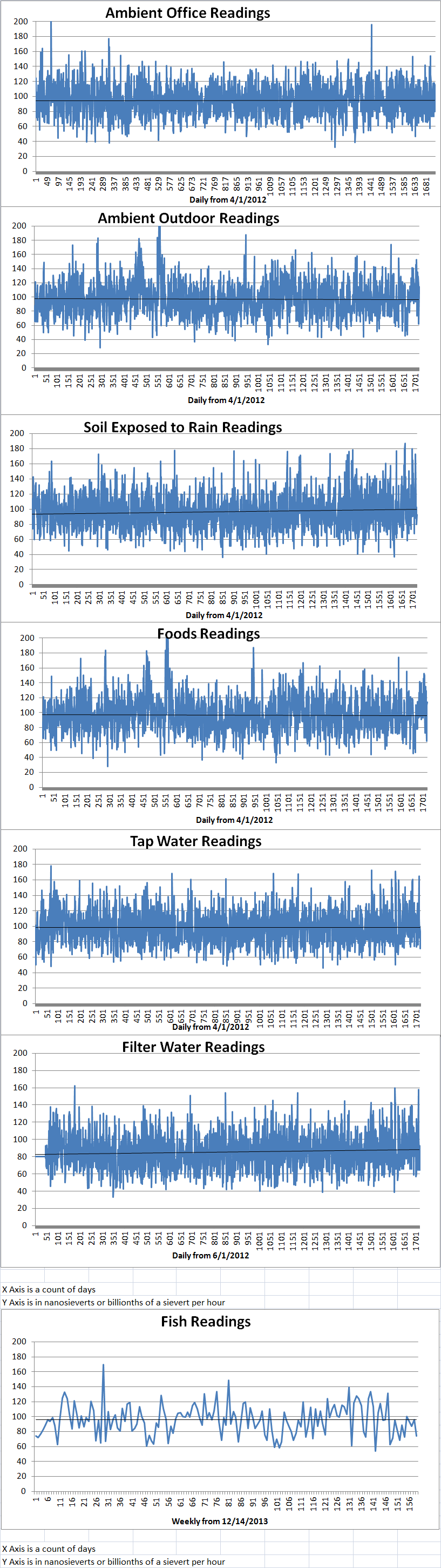
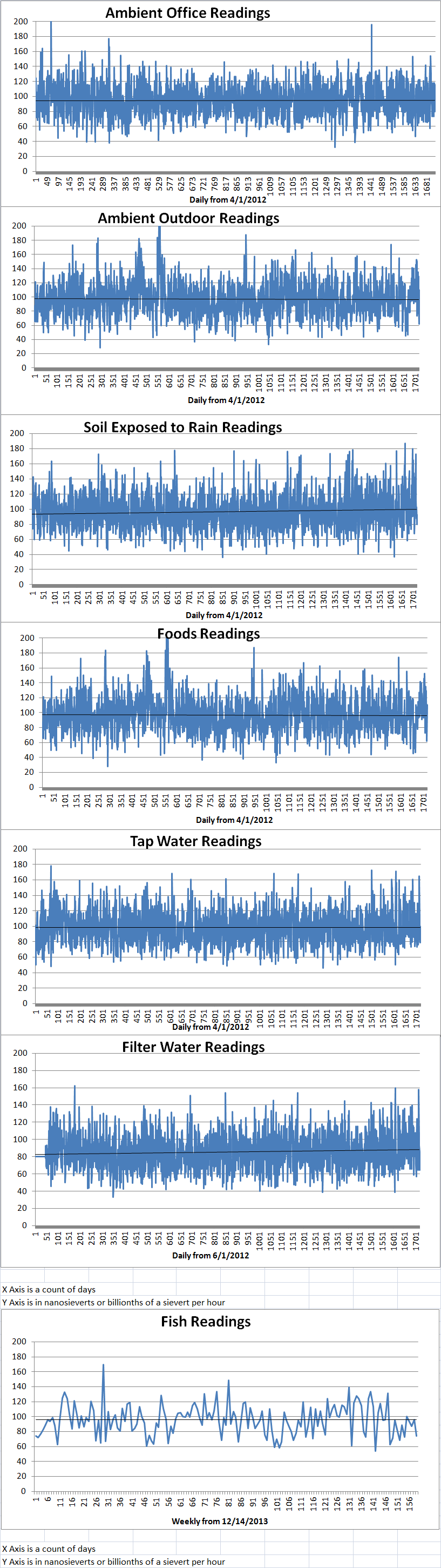
Ambient office = 115 nanosieverts per hourAmbient outside = 147 nanosieverts per hourSoil exposed to rain water = 130 nanosieverts per hourCarrot from Central Market = 99 nanosieverts per hourTap water = 109 nanosieverts per hourFilter water = 91 nanosieverts per hour -
Geiger Readings for Mar 11, 2017
Ambient office = 70 nanosieverts per hourAmbient outside = 114 nanosieverts per hourSoil exposed to rain water = 109 nanosieverts per hourBartlett pear from Central Market = 70 nanosieverts per hourTap water = 108 nanosieverts per hourFilter water = 102 nanosieverts per hourDover sole – Caught in USA = 60 nanosieverts per hour -
Radioactive Waste 221 – Chemistry of Uranium And Thorium Compounds
For the past eight years, chemists in the United Kingdom at the University have been developing our understanding of how uranium and thorium interact with elements from around the periodic table to potentially help improve the creation of nuclear fuel and also the selective extraction of spent uranium for nuclear waste clean-up.
Stephen Liddle, Professor of Inorganic Chemistry and Royal Society University Research Fellow at the University of Manchester has been studying the chemistry of molecular depleted uranium chemistry since 2009. In 2012, his team created what is referred to as a “trophy molecule” of uranium nitride. Chemists had been trying to create a uranium nitride compound that was stable at room temperature for decades. The compound created by the Liddle team might possibly lead to a new form of nuclear fuel that has superior high densities, melting points, and thermal conductivities than that in existing mixed oxide nuclear fuel.
Following his work on uranium nitride, Liddle and his team worked on the development of a process for removing uranium from nuclear waste. He said that “We need to reduce the volume of nuclear waste to make it easier to handle, and process it to remove benign elements or separate the high-level waste from low-level waste. This latest study looked at how soft elements such as arsenic interact with uranium—arsenic could in principle be used in organic molecules that bond to metal atoms and improve extraction processes.” Professor Liddle went on to say “There is currently a lot of interest in using organic molecules to extract, selectively, metal ions from the ‘soup’ of nuclear waste and fish out the ‘bad’ ones and leave the rest behind. This requires an understanding of chemical bonding and how the organic extractants bind to different metals. We can then exploit this knowledge to achieve separation by having them selectively bind to one type of metal and remove it from the soup.”
There is mounting evidence that molecules that contain what are called “soft donor” atoms for metals might be best for cleaning up nuclear waste. Arsenic is a “soft donor” and easily forms stable compounds with many minerals. Dr. Liddle’s group has created a variety of molecular complexes to test exactly how arsenic and phosphorous bind to metals. These compounds include uranium-phosphorus, uranium-arsenic, thorium-phosphorus, and thorium-arsenic
Elizabeth Wildman, a PhD student in Dr. Liddle’s group at the University of Manchester recently discovered a way to produce grams of a compound of arsenic and thorium that previously had only been produced in tiny quantities. Previous samples had been prepared at extremely low temperatures. Wildman’s process was much simpler and did not require such extreme temperatures. Her samples were stable at room temperatures. Wildman said “Nuclear power could potentially produce far less carbon dioxide than fossil fuels, but the long-lived waste it produces is radioactive and needs to be handled appropriately.”
These breakthroughs at the University of Manchester will allow chemists to easily prepare and study samples of uranium and thorium compounds. Eventually, as the chemistry of such compounds is studied, better ways of producing nuclear fuels and extracting uranium from radioactively contaminated water may be discovered.
University of Manchester – School of Chemistry:
-
Geiger Readings for Mar 10, 2017
Ambient office = 95 nanosieverts per hourAmbient outside = 100 nanosieverts per hourSoil exposed to rain water = 91 nanosieverts per hourCelery from Central Market = 114 nanosieverts per hourTap water = 71 nanosieverts per hourFilter water = 65 nanosieverts per hour -
Nuclear Weapons 257 – North Korea’s Recent Missile Launches And Attempts To Sell Nuclear Materials Increase International Concern
North Korea has been in the news lately because of their test launching of intermediate-range ballistic missiles that might be able to carry nuclear warheads. As other nations criticized the tests, N.K. issued increasingly belligerent responses. N.K. has been accused in the past of supply or offering to supply nuclear technology to other nations in defiance of international treaties opposing the spread of nuclear weapons. Now it has been reported that N.K. has also tried to sell nuclear materials on the international black market.
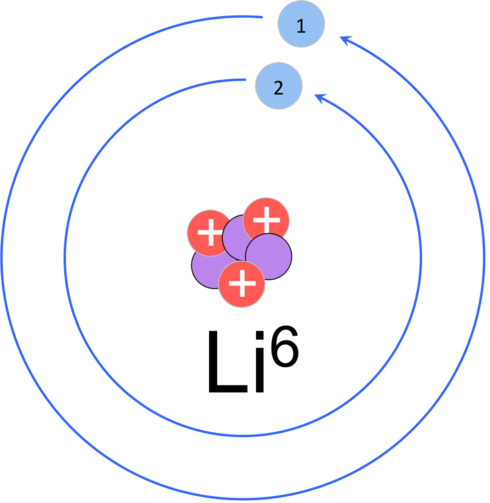
U.N. investigators tracking the N.K. nuclear weapons program have announced that Green Pine, which is Pyongyang’s “primary arms dealer and main exporter of goods and equipment related to ballistic missiles” tried to sell enriched lithium metal to unspecified buyers last year. Lithium has nonmilitary uses such as the manufacture lubricants and medicines. The most popular type of batteries for consumer electronics are lithium ion batteries. N.K. has large deposits of lithium which is about eight percent lithium-6. The lithium for sale by Green Pine had a high concentration of lithium-6 which is useful in the manufacture of nuclear bombs.
Lithium ore is processed to produce a much greater concentration of lithium-6. Lithium-6 can be used to create tritium, the radioactive isotope of hydrogen. Tritium can produce a flood of neutrons that can amplify the explosive power of a nuclear bomb. This allows smaller amounts of plutonium or uranium to be used in bombs that have greater power than bigger bombs without tritium neutron injection. N.K. is known to be working on reducing the size of their warheads for launch on their missiles. In addition to making tritium, lithium-6 can be used directly in the manufacture of nuclear bombs including the thermonuclear variety.
In light of the recent N.K. missile launches and belligerent statements with respect to nuclear war, the announcement of the attempted sale of lithium-6 to another nation has increased the concern of the U.S and South Korea. The U.S. and South Korea are planning joint military exercises in the near future. These military exercises are being portrayed as preparations for invasion by N.K. The N.K. government says that their nation must be prepared for all out war including the use of nuclear weapons. The U.S. has responded that any attack on S.K. will result in overwhelming retaliation by the U.S. The U.S. President has ordered B-1 and B-52 bombers to S.K. to participate in the exercises. Both of these bombers are designed to carry nuclear bombs.
The problem with threatening to use nuclear weapons to retaliate against N.K.’s use of nuclear weapons is the fact that the Korean peninsula is rather small. Any nuclear explosion on the peninsula would scatter fallout over the whole peninsula regardless of whether the North or the South caused the explosion. Multiple nuclear explosions could render the whole peninsula uninhabitable. There would be millions killed immediately and millions more would die from radiation exposure. Millions would flee into China causing a massive refugee crisis.
China is about the only friend that N.K. has in the family of nations. When China criticized N.K. for its recent missile launch, N.K. responded with hostile rhetoric. The ruler of N.K. is unstable personally and his grip on power is also unstable. There is a fear that as tensions on the Korean peninsula increase, there might come a point where the N.K. ruler decides that a desperate attack on S.K. and other neighbors might be his only choice.
-
Geiger Readings for Mar 09, 2017
Ambient office = 80 nanosieverts per hourAmbient outside = 91 nanosieverts per hourSoil exposed to rain water = 92 nanosieverts per hourCurrant from Central Market = 110 nanosieverts per hourTap water = 79 nanosieverts per hourFilter water = 65 nanosieverts per hour -
Nuclear Reactors 264 – Japan Should Reconsider Its Dedication To Nuclder Power
Shinzo Abe was first elected Prime Minister of Japan in 2006. He made nuclear power and export of nuclear technology a cornerstone of his campaign. In March of 2011, a tsunami caused by an earthquake flooded a nuclear power plant on the coast of Fukushima prefecture in northeastern Japan. Three reactors melted down and there was an explosion that severely damaged a fourth reactor. Negligence on the part of the Tokyo Electric Company was the cause of the disaster. All nuclear reactors in Japan were shut down following the event. Following his reelection to be Prime Minister in 2012, Abe still insisted that Japan should embrace nuclear power and export nuclear technology.
It has now been six years since the disaster. Radioactive water from the site is still flowing into the Pacific Ocean. The reactors which melted down are still too radioactive to allow workers to find out exactly where the melted fuel is. Robots that are sent in to send back pictures keep being destroyed by the radiation. There have been projections that the workers will be able to remove the nuclear fuel from the destroyed reactors starting in 2021 but that is probably wishful thinking.
It will take decades of decontamination, decommissioning and reconstruction to deal with the aftermath of the disaster. The initial estimate for recovery from the disaster has doubled from nine billion dollars to eighteen billion dollars and will probably rise even more. New regulations have been put into place to pass the cost of recovery along to the rate-payers in Japan. Last year the energy market was opened to competition but the claim that nuclear power is competitive is obviously false.
Only a few of the Japanese reactors have been restarted after all viable reactors were upgraded to match new regulations. Although the government has stated that it will reduce Japanese dependence on nuclear power in the future, the nuclear power industry intends to start at least twenty-six of the fifty-six reactors that were operating in Japan before the disaster. Analysts claim that Japan will not experience a power shortage if none of the reactors are restarted. There is widespread public opposition to restarting all these reactors.
Supporters of nuclear power in Japan point to the need to reduce carbon emissions because of global warming. However, even with the reactors being shut off since the disaster, Japan’s carbon emission have been falling in the past few years. Japan has met its obligations for carbon emission reduction under the Paris Accords without nuclear power. With effective conservation and renewable energy, Japan can continue to reduce carbon emission even more.
The cost of renewable energy sources such as solar and wind are falling and investors are moving their money to renewable energy. As renewables are rising, the demand for nuclear power in developed countries is falling. Major nuclear corporations such as Toshiba in Japan and Areva in France have experienced serious financial problems with their nuclear products and services. It would be wise for Japan to take heed of these developments and to abandon nuclear power altogether.
Japanese Prefectures: