Part 1 of 3 Parts
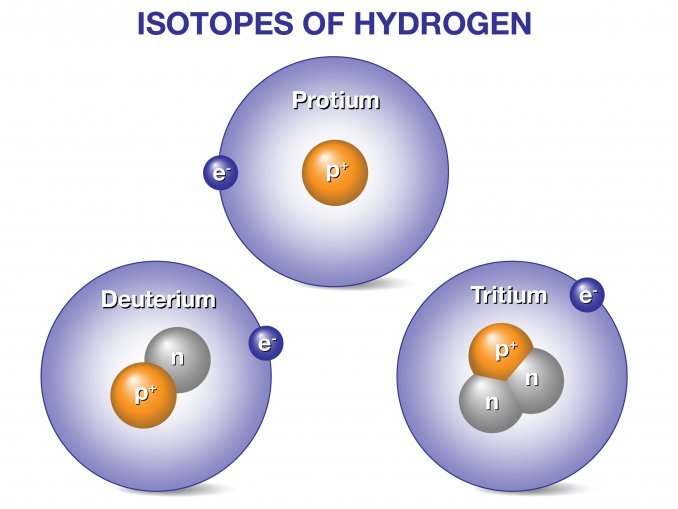
Tritium is an isotope of hydrogen that is very similar to the other two hydrogen isotopes. Protium is another name for the most familiar and most common hydrogen isotope which contains one proton in its nucleus. Deuterium is the isotope which contains a neutron as well as a proton in its nucleus. Tritium has two neutrons in its nucleus and is an unstable radioactive isotope which decays into helium-3 with a half-life of about twelve years. Most of the naturally occurring tritium on Earth is generated by interactions between fast neutrons from space and atmospheric nitrogen.
Tritium has been in the news recently because the Japanese government has announced that it going to release treated water from the Fukushima Daiichi nuclear power plant which melted down in 2011. Many people are raising the question of how much tritium is ‘too much’ and what is likely to be seen when this tritium contaminated ground water is released into the Pacific Ocean.
With respect to the risk of exposure to radioactivity, the Linear-Non-Threshold (LNT) is the most common and respected model. The model claims that there is a linear match between exposure to radiation and the probability that the patient may develop cancer or other negative side-effects that could be caused by such exposure.
Multiple recent studies suggest that reality is not as simple as the LNT model. There are varying effects from different types of radiation on different parts of the body and the ability of an exposed body’s ability to repair damage to cells. The actual impact of radiation on mouse models shows no cytotoxicity or genotoxicity in the spleen after exposure to beta radiation from tritium exposure. The studies also showed no upregulation of the immune system after exposure to low-dose radiation (LDR). The current evidence in the literature indicates that LDR may exert a positive influence on the immune system of a human body. If this proves to be true, it could have important effects on our understanding of cancer treatments.
These studies also provide some indication of just how fearful we should be of radiation our environment. For instance, levels of cesium-137, potassium-40 and hydrogen-3 have been used to track the age of wine. This can be used in fraud cases where a younger wine is substituted for an older wine in order to increase the price. They found that a Bulgarian wine from 2001 had cesium levels of less than fifteen percent of a single Bq/L. (Bq stands for one nuclear decay event per second and L stands for Liter so Bq/L stands for one nuclear decay per second per liter.) For comparison, the same vintage from 1968, the year of the nearby Chernobyl nuclear power plant disaster, contained over forty-four Bq/L.
Tritium levels fluctuated between seven and sixty-three Bq/L and potassium-40 levels varies between fifteen and twenty Bq/L during the same time span. Tritium levels in rainwater average about half a Bq/L and in surface water it varies between about a third of a Bq/L and eleven tenths of a Bq/L.
Please read Part 2 next