Vitrification is a process of combining radioactive materials with other substances including silicon to yield a glass log that can be permanently disposed of in a geological repository, insuring that the radioactive materials will not be leached out of the log by ground water. If vitrification is being used to dispose of spent nuclear fuel, one of the first steps is to dissolve the cladding materials of the fuel rods. This step often utilizes sulfuric acid or H2SO4. The bisulfate ion or HSO4 can remain in the solution and cause problems with further stages of processing.
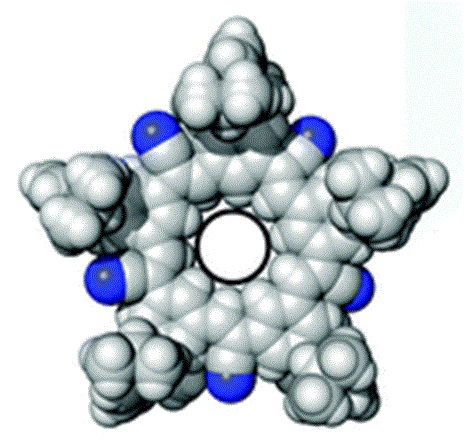
Researchers at Indiana University at Bloomington created a cyanostar macrocycle. A macrocycle is a ring of atoms that contains eight or more. The cyanostar is a five sided or star shaped molecule that has an affinity for anions or negatively charged molecular fragments or ions. These cyanostar macrocycles are easy to synthesize and were developed because they had potential use in trapping various types of pollutants inside the ring including borates, chlorates and phosphates.
The Indiana researchers have discovered a "supermolecule" that may be able to aid in the removal of bisulfate from the vitrification solution. The supermolecule is a combination of two anions or monomers of bisulfate that form what is called a dimer. Monomers are molecules that can be bound with identical monomers to form a chain that is referred to as a polymer or "many monomers". When only two monomers are joined, it is called a dimer or "two monomers."
Dimers composed of two anions were thought to be impossible because the two negative ions should repel each other according to standard theories of electrochemistry. However, there are short range attractions that make the combination possible because they overcome the expected long range repulsion between the two negatively charged ions.
Normally, monomers bond with what is called a covalent bond. This is created when an electron from each of the bonding monomers interact to create the bond. However the new supermolecular found by the researchers is based on many weak non-covalent bonds connecting two monomers. The bisulfate monomers mentioned above connect via a self-complementary, anti-electrostatic hydrogen bond.
The Indiana researchers were trying to trap a single bisulfate monomer inside the cyanostar as proof of concept for using the cyanostar to capture bisulfate left over from sulfuric acid in processing spend nuclear fuel for disposal. They were surprised to find two bisulfate monomers inside each ring during their test. Trapping a dimer made of two bisulfate monomers inside each ring would be twice as effective as they were expecting for bisulfate removal from a vitrification solution. Vitrification is a promising method of encapsulating spent nuclear fuel waste for disposal and any improvement in the process is welcome.
These cyanostar macrocycles will also be able to remove harmful phosphates, borates and chlorates from the environment. Phosphates from fertilizers run off land into bodies of water and cause environmental havoc by spawning massive algae blooms and killing huge numbers of fish.
Artist's concept of cyanostar: