Part 1 of 3 Parts
Although India has not released any official statements about the size of its nuclear arsenal, recent estimates suggest that India has one hundred and seventy-two nuclear weapons and has produced enough weapons-grade plutonium for up to two hundred nuclear weapons. In 1999, India was estimated to have eighteen hundred pounds of separated reactor-grade plutonium, with a total amount of eighteen thousand three hundred pounds of civilian plutonium, enough for approximately one thousand nuclear weapons. India has conducted nuclear weapons tests in a pair of series namely Pokhran I and Pokhran II.
India’s nuclear program can trace its origins to March 1944 and its three-stage efforts in technology were established by Homi Jehangir Bhabha when he founded the nuclear research center, the Tata Institute of Fundamental Research. India first tested a nuclear device in 1974 (code-named “Smiling Buddha”), under Prime Minister Indira Gandhi as a peaceful nuclear explosion.
India’s second nuclear-capable ballistic missile submarine was deployed late last month. The nuclear-powered sub is called INS Arighaat which means “Destroyer of the Enemy” in Sanskrit. This is a move the government says strengthens its nuclear deterrent as India casts a wary eye at both China and Pakistan.
But India is still playing catch-up when compared with China, as the People’s Liberation Army grows its fleet amid simmering tensions along their shared border.
The new sub will “help in establishing strategic balance” in the region according to Indian Defense Minister Rajnath Singh. He mentioned the new sub at an August 29 commissioning ceremony at Visakhapatnam naval base, the headquarters of India’s Eastern Naval Command on the Bay of Bengal coast.
That balance currently tilts in favor of China which has the world’s largest navy by numbers. It includes six operational Jin-class nuclear-powered ballistic submarines that outclass India’s two – Arighaat and its predecessor in the same class, INS Arihant – in firepower.
The Chinese subs can carry a dozen ballistic missiles with ranges of at least 4,970 miles. They have the ability to carry multiple nuclear warheads, according to the Missile Defense Advocacy Alliance, a non-profit organization promoting the development and deployment of missile defense for the United States and its allies.
Arighaat and Arihant are both three hundred and sixty-six feet long with a six-thousand-ton displacement They each carry K-15 Sagarika ballistic missiles that can be launched from four vertical launch tubes. However, the range of the nuclear-tipped K-15 is thought to be only around 466 miles, limiting the targets that can be struck from the Indian Ocean. This information was reported in an analysis by the open-source intelligence agency Janes,
Carl Schuster is a former director of operations at the US Pacific Command’s Joint Intelligence Center. He said, “The INS Arihant-class can barely reach Chinese targets along the eastern Sino-Indian border from the coastal waters of northern Bay of Bengal, which is dangerously shallow for a submarine.”
The de facto border between India and China is known as the Line of Actual Control. It has been a longtime flashpoint between the two. Troops clashed there in 2022 and in 2020, when hand-to-hand fighting between the two sides resulted in the deaths of at least 20 Indian and four Chinese soldiers in Aksai Chin.
Please read Part 2 next
Blog
-
Nuclear Weapons 874 – India Launches Another Nuclear Capable Nuclear-Powered Submarine – Part 1 of 3 Parts
-
Nuclear News Roundup Oct 14, 2024
Westinghouse extends FEED contract for new Kozloduy units world-nuclear-news.org
US nuclear regulator kicks off review on Three Mile Island restart Investing.com
NextEra considers nuclear restart in Iowa, while renewable deals swell finance.yahoo.com
Mississippi’s new PSC energized by nuclear power, tepid over renewables mississippitoday.org
-
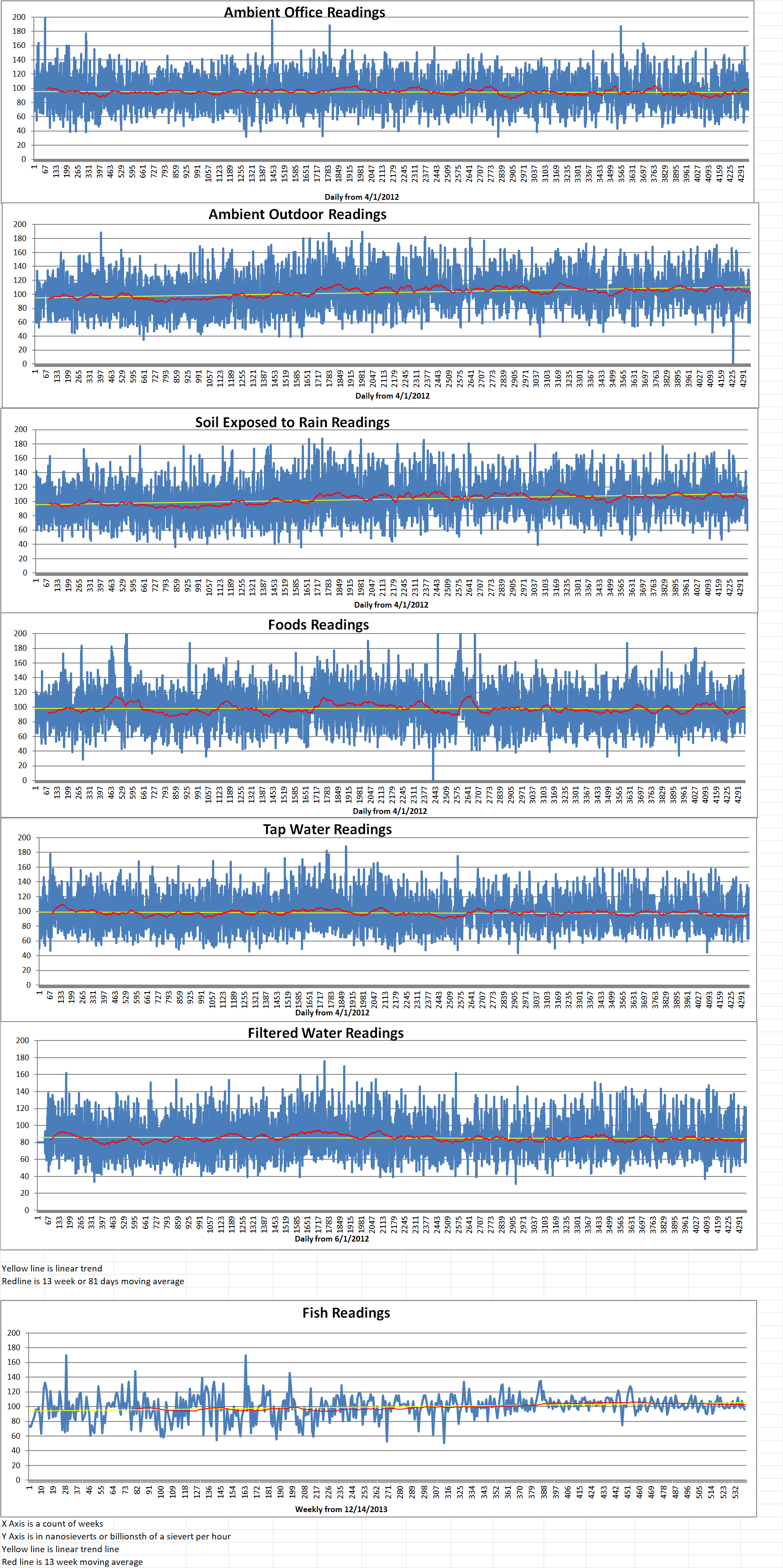
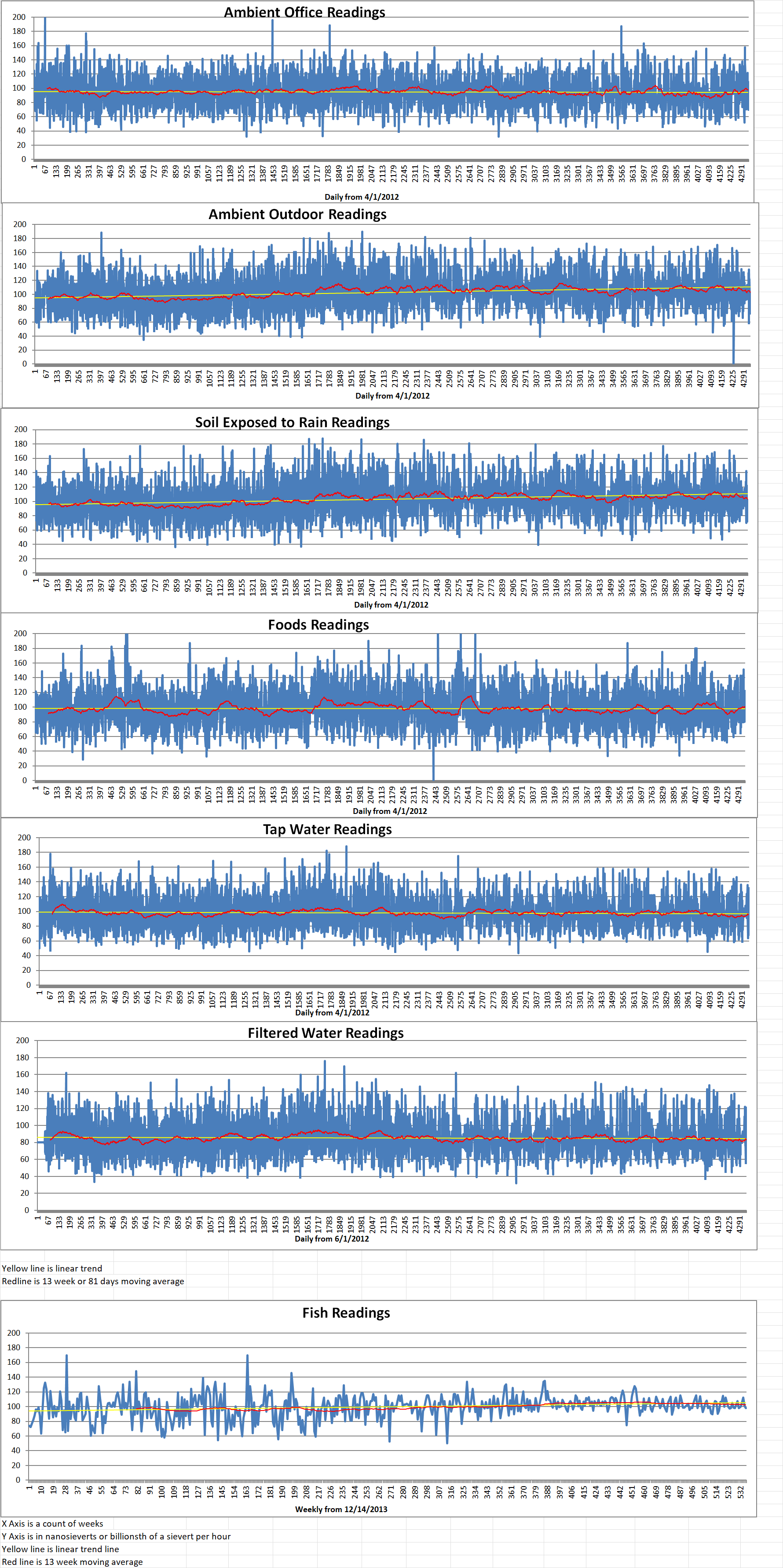
Geiger Readings for Oct 14, 2024
Ambient office = 84 nanosieverts per hour
Ambient outside = 91 nanosieverts per hour
Soil exposed to rain water = 100 nanosieverts per hour
Green onion from Central Market = 73 nanosieverts per hour
Tap water = 115 nanosieverts per hour
Filter water = 109 nanosieverts per hour
-
Nuclear News Roundup Oct 13, 2024
Jamaica signs MoU to advance nuclear adoption world-nuclear-news.org
Orano suspends operations at Arlit world-nuclear-news.org
Decommissioning permit granted for Brokdorf plant world-nuclear-news.org
Mochovce 4 passes safety system tests world-nuclear-news.org
-
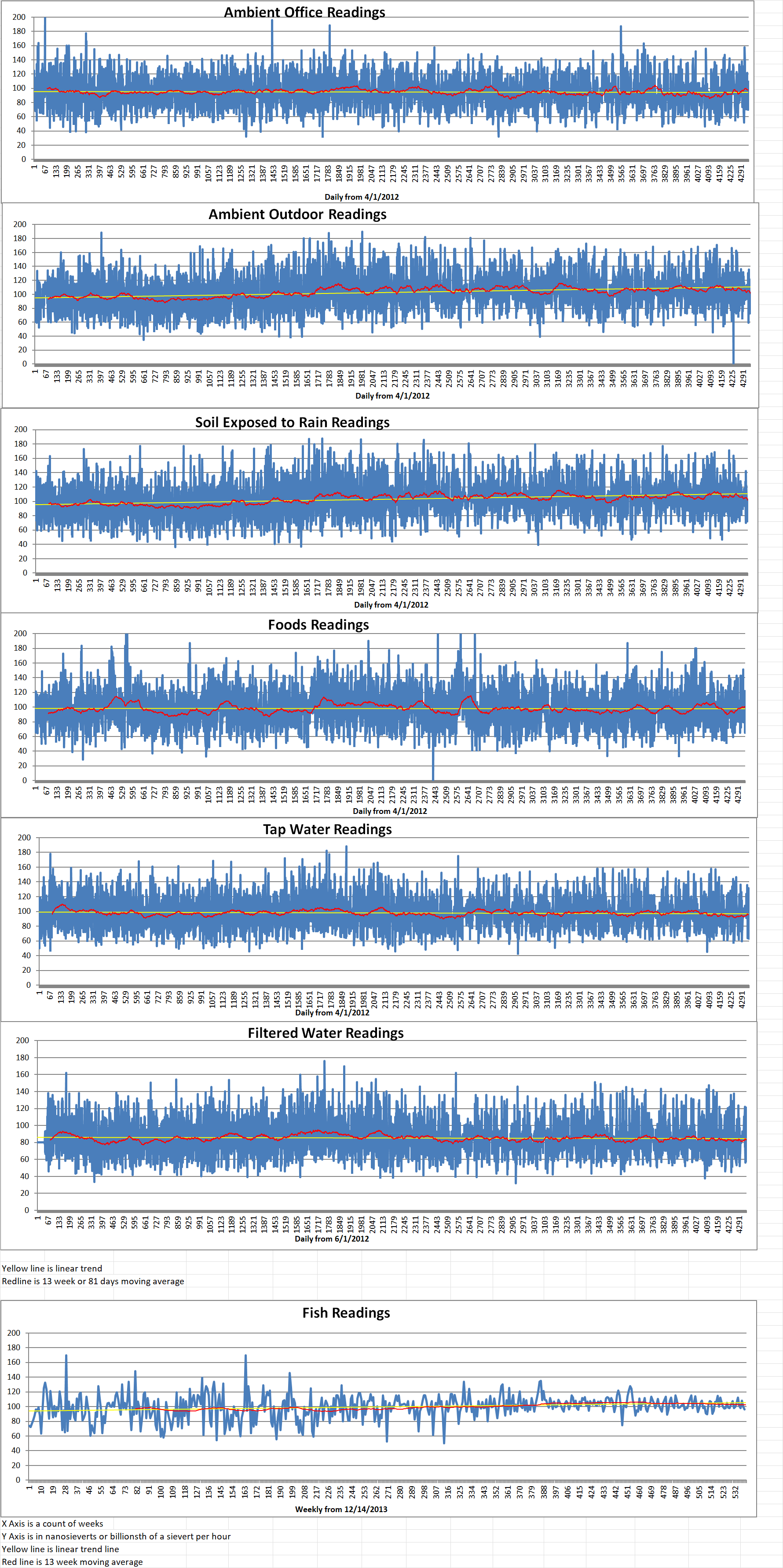
Geiger Readings for Oct 13, 2024
Ambient office = 66 nanosieverts per hour
Ambient outside = 125 nanosieverts per hour
Soil exposed to rain water = 124 nanosieverts per hour
Blueberryfrom Central Market = 78 nanosieverts per hour
Tap water = 93 nanosieverts per hour
Filter water = 83 nanosieverts per hour
-
Nuclear News Roundup Oct 12, 2024
Navy names newest nuclear-powered submarine USS Atlanta ajc.com
US Submarine Earns Highest Military Honor for Spying on America’s Enemies newsweek.com
U.S. companies exposed to the nuclear energy trend seekingalpha.cm
US power grid added battery equivalent of 20 nuclear reactors in past four years theguardian.com
-
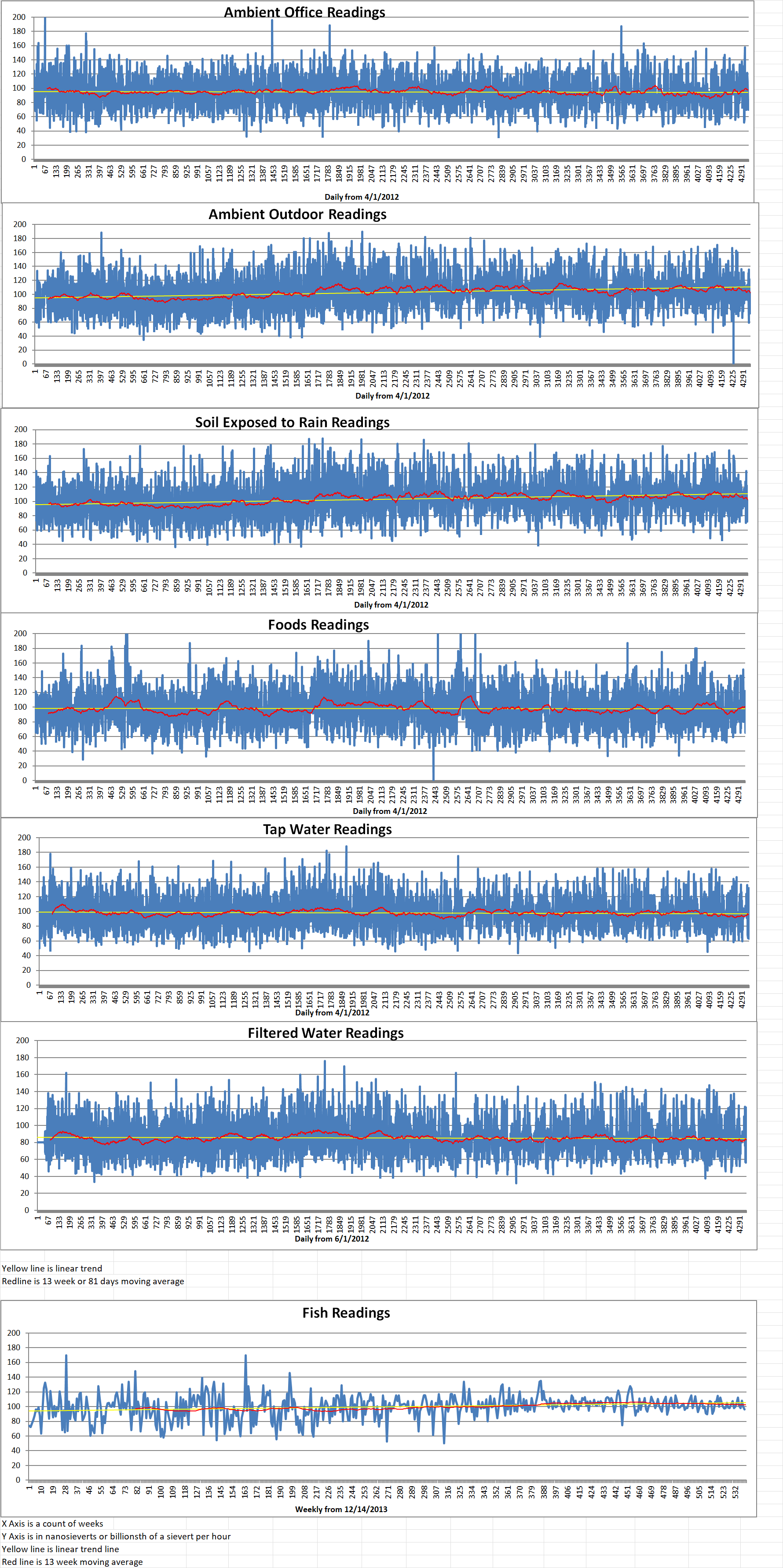
Geiger Readings for Oct 12, 2024
Ambient office = 76 nanosieverts per hour
Ambient outside = 109 nanosieverts per hour
Soil exposed to rain water = 112 nanosieverts per hour
Avocado from Central Market = 87 nanosieverts per hour
Tap water = 110 nanosieverts per hour
Filter water = 100 nanosieverts per hour
Dover Sole from Central = 103 nanosieverts per hour
-

Nuclear Fusion 83 – New Method Discovered To Allow Better Separation Of Hydrogen Isotopes
Elementary hydrogen has three isotopes: protium, deuterium, and tritium. These isotopes play a critical role in hydrogen fuel production, nuclear fusion, and the development of advanced pharmaceuticals.
However, it’s very difficult to isolate these isotopes at room temperature. This is because they have similar sizes and shapes. In addition, each of them has one proton and one electron, leading to similar chemical and thermodynamic properties. The methods currently used for separating hydrogen isotopes are resource-intensive as they require extreme conditions to work.
Knut Asmis is a chemistry professor at Leipzig University. He said, “It has been known for almost 15 years that porous metal-organic frameworks can, in principle, be used to purify and separate hydrogen isotopes. However, this has only been possible at very low temperatures, around minus 200 degrees Celsius—conditions that are very costly to implement on an industrial scale.”
Asmis and his colleagues recently published a report that provides important insights into how hydrogen isotopes can be isolated at room temperature and at a low cost. Their research reveals that the secret is water ligand.
When porous metal ions such as Cu+ interact with hydrogen under extreme conditions, they perform selectively absorption of the different isotopes. The study authors note that “Adsorption is a process by which atoms, ions, or molecules from a gas or liquid adhere to a solid, often porous, surface”.
During their study, the researchers discovered that when water molecules are used as ligands with the copper ion, the metal becomes better at attracting and holding hydrogen molecules. In addition, the resulting copper-water complex is better at distinguishing the energy difference in the bonds between H2 (regular hydrogen) and D2 (heavy hydrogen) compared to bare copper.
The authors continue that “Combining experimental and computational methods, we demonstrate a high isotopologue selectivity in dihydrogen binding to Cu+(H2O), which results from a large difference in the adsorption zero-point energies (2.8 kJ mol−1 between D2 and H2, including an anharmonic contribution of 0.4 kJ mol−1).”
Unlike bare copper ions, the copper water complex doesn’t need large amounts of energy to achieve hydrogen isotope isolation. This suggests that it might lead to more efficient, less resource-intensive, and highly cost-effective ways of obtaining hydrogen isotopes.
The research shows that porous metal complexes with water ligands are promising candidates for hydrogen isotope isolation. It also suggests that metal-water complexes might be used to study chemical reactions that take place at particular sites in large systems.
The authors added that “These systems are ideal model complexes for gas-phase studies of the chemistry at individual active sites as they occur in framework materials.”
Asmis and his team also carried out spectroscopy and complex quantum calculations to understand the interaction between the hydrogen isotopes in detail. Their findings may reveal more practical ways of selective absorption of isotopes.
Thomas Heine is one of the study authors and an expert in theoretical chemistry at Technische Universität Dresden. He said, “For the first time, we have been able to show the influence of the individual atoms of the framework compounds on adsorption. We can now optimize them in a targeted manner in order to obtain materials with high selectivity at room temperature.” -
Nuclear News Roundup Oct 11, 2024
Argentina ramping up radioisotope production world-nuclear-news.org
Orano sets up working groups for fast reactor fuels world-nuclear-news.org
SMRs becoming a reality, says Grossi world-nuclear-news.org
Partnership formed for UK Integral Molten Salt Reactor project world-nuclear-news.org
-
Geiger Readings for Oct 11, 2024
Ambient office = 74 nanosieverts per hour
Ambient outside = 91 nanosieverts per hour
Soil exposed to rain water = 96 nanosieverts per hour
Asparagus from Central Market = 151 nanosieverts per hour
Tap water = 91 nanosieverts per hour
Filter water = 83 nanosieverts per hour
