The primary uranium minerals in commercial ores are uraninite (UO2), pitchblende (U3O8), coffinite (U(SiO4), brannerite (UTi2O6), davidite ((REE)(Y,U)(Ti,Fe3)20O38) and thucholite (Uranium-bearing pyrobitumen). There are a number of other common uranium minerals which form hydrated crystals incorporating water molecules.
The mineralogy of the host minerals, the reduction-oxidation potential of the uranium mineral and the porosity which determines water infiltration are important factors in the formation of uranium deposits. Since uranium is highly soluble, it can be easily moved around by the flow of water underground. This contributed to the variety of places and manners in which uranium may accumulate. The way in which uranium interacts with other elements and compounds in melted rock also influences its distribution.
Combinations of surface weathering, sedimentation, diagenetic, magmatic and hydropthermal geological processes mentioned in a previous post produce fifteen general types of uranium deposits.
The richest uranium ore deposits are found near unconformities. An unconformity is a break in between two layers of rock that have been laid down at different times. In the case of uranium deposits, the two layers are a quartz rich sedimentary layer and a metamorphic layer has been altered by heat and pressure. These deposits were formed between two billion five hundred million years ago and five hundred million years ago.
The second best uranium ore deposits form in sedimentary deposits on continental shelves and freshwater areas such as river deltas, lakes, etc. In an oxygen rich environment, the uranium dissolves and then moves with the water. When it encounters an oxygen poor or reducing environment, it precipitates out of solution.
Tabular deposits occur parallel to groundwater flow in sandstone. The ores are rich but the deposits are small.
Roll front uranium deposits form when ground water dissolves the uranium in sandstone and, after flowing underground, collides with some sort of organic matter rich in carbon. The uranium precipitates out at the “front” when the water encounters the organic material.
Basal channel deposits form from moving ground water like the tabular and roll front deposits, but the deposition occurs along channels of moving surface water such are rivers. When the water evaporates along desert margins or in shallow saline ponds, the uranium is deposited.
Quartz-pebble conglomerate deposits are created by the separation and movement of particles of uranium in flows of surface water and their deposit in river beds, river deltas and lakes. These deposits generally contain large quantities of low grade ore
Breccia complex deposits contain uranium along with iron oxide, copper, gold, silver and rare earth elements. Hydrothermal processes enriched the uranium content of the quartz-hematite breccias.
Vein deposits are uranium minerals filling in cracks, veins, fractures and breccias in steeply dipping fault systems. Magmatic processes in molten rock create the veins and later hydrothermal activity can concentrate the uranium. Some veins contain a variety of other metals in combination with the uranium.
Intrusive uranium deposits form when magma is forced into older rocks deep within the Earth’s crust.
Marine sedimentary deposits of phosphorite (which contain large amounts of phosphorus) sometimes contain uranium.
Collapsed breccia pipe deposits are created when vertical cylindrical cavities formed by groundwater dissolving limestone are filled with fragments of rock when they collapse. Uranium fills cavities and coats other rocks.
Volcanic deposits of uranium may be formed by magmatic processes in the molten rock or later mineralization by groundwater and chemical processes. Such deposits are usually small with low grade ore.
Surface deposits can form in peat bogs, karst caverns and in soil from the weathering of shallow sedimentary deposits of uranium.
Metasomite deposits are the result of uranium minerals being distributed in rocks that have been subjected to sodium metasomatism which is chemical alteration by hot subsurface solutions of sodium.
Metamorphic deposits were laid down by sedimentary or magmatic processes and then remained unaltered by any other processes.
Lignite is a soft brown young coal derived from wood. Some deposits contain significant amounts of uranium minerals.
Black shale deposits form in oxygen-free submarine sedimentation processes. The uranium is not mineralized by organic materials due to the lack of oxygen. These deposits are considered very low grade ores.
There are many other types of uranium minerals but these fifteen types constitute the pool from which uranium ores are chosen for extraction.
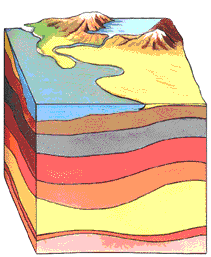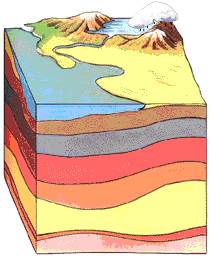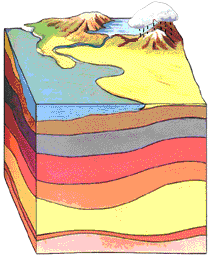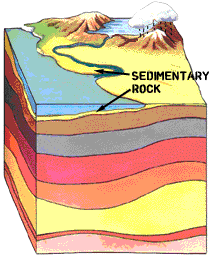
Sedimentary layers: